
��14�֣���ѧ����Դ����������������ʮ����Ҫ�����á�
��1���̲��ں��Ŀ�ȼ���Ǹ�ѹ���γɵ��������ļ���ˮ������壮����֮Ϊ��δ����Դ������25�桢101 kPa�£�1g������ȫȼ�����ɺ�Һ̬ˮʱ����55.6 kJ������ȼ�յ��Ȼ�ѧ����ʽΪ ______����ͬ�����£�356 g��ȼ��������ʽΪCH4��9H2O��Mr��178���ͷŵļ���������ȫȼ������CO2��Һ̬ˮ���ų�������Ϊ_______kJ��
��2��������(CH3OCH3)����ɫ���壬����Ϊһ��������Դ��������ࡢ��Ч���������ܡ��ɺϳ���(���ΪH2��CO��������CO2)ֱ���Ƹ������ѣ����е���Ҫ���̰��������ĸ���Ӧ��
�״��ϳɷ�Ӧ��
����CO��g��+2H2��g���TCH3OH��g�� ��H1����90.1kJ?mol-1
����CO2��g��+3H2��g���TCH3OH��g��+H2O��g�� ��H2����49.0kJ?mol-1
ˮú���任��Ӧ������CO��g��+H2O��g���TCO2��g��+H2 ��g�� ��H3����41.1kJ?mol-1
�����Ѻϳɷ�Ӧ��������2CH3OH��g���TCH3OCH3��g��+H2O��g����H4����24.5kJ?mol-1
�ٷ��������Ѻϳɷ�Ӧ(iv)����COת���ʵ�Ӱ��___________________________________��
����H2��COֱ���Ʊ������ѣ���һ����Ϊˮ���������Ȼ�ѧ����ʽΪ��__________________�����ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ��_________________________________��
��3��������ֱ��ȼ�ϵ�ؾ��������졢Ч�ʸߵ��ŵ㡣�������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ______________________��һ�������ѷ��Ӿ����绯ѧ���������Բ���________���ӵĵ�����
��1��2O2��g��+CH4��g���TCO2��g��+H2O��l�� ��H����889.6kJ/mol 1779.2
��2�������ļ״����ٽ��״��ϳɷ�Ӧ����ƽ�����ƣ�COת�����������ɵ�H2O��ͨ��ˮú���任��Ӧ�������IJ���CO
��2CO��g��+4H2��g����CH3OCH3��g��+H2O��g�� ��H����204.7kJ?mol-1
�÷�Ӧ���������٣�ѹǿ����ʹƽ�����ƣ�CO��H2ת��������CH3OCH3�������ӣ�ѹǿ����ʹCO��H2Ũ�����ӣ���Ӧ��������
��3��CH3OCH3��16OH����12e����2CO32����11H2O��12
���������������1��1g������ȫȼ�����ɺ�Һ̬ˮʱ����55.6 kJ����16g���鼴1mol������ȫȼ�����ɺ�Һ̬ˮʱ����55.6 kJ��16��889.6kJ����˼���ȼ�յ��Ȼ�ѧ����ʽΪ2O2��g��+CH4��g���TCO2��g��+H2O��l�� ��H����889.6kJ/mol��356 g��ȼ��������ʽΪCH4��9H2O��Mr��178���м����������356g�� ��32g��������2mol���飬�����ͷŵļ���������ȫȼ������CO2��Һ̬ˮ���ų�������Ϊ889.6kJ/mol��2mol��1779.2kJ��
��32g��������2mol���飬�����ͷŵļ���������ȫȼ������CO2��Һ̬ˮ���ų�������Ϊ889.6kJ/mol��2mol��1779.2kJ��
��2���ٶ����Ѻϳɷ�Ӧ����������COת���ʵ�Ӱ�죬���ļ״����ٽ��״��ϳɷ�Ӧ����CO��g��+2H2��g���TCH3OH��g��ƽ�����ƣ�COת�����������ɵ�H2O��ͨ��ˮú���任��Ӧ����CO��g��+H2O��g���TCO2��g��+H2 ��g�����IJ���CO���ʴ�Ϊ�����ļ״����ٽ��״��ϳɷ�Ӧ����ƽ�����ƣ�COת�����������ɵ�H2O��ͨ��ˮú���任��Ӧ�������IJ���CO��
�ڢ�CO��g��+2H2��g���TCH3OH��g����H1=-90.1kJ?mol-1������2CH3OH��g���TCH3OCH3��g��+H2O��g����H4=-24.5kJ?mol-1�����ݸ�˹���ɢ��2+���õ���2CO��g��+4H2��g��=CH3OCH3+H2O��g�� ��H����204.7kJ?mol-1���÷�Ӧ�����������С�ķ�Ӧ������ѹǿƽ��������У���Ӧ��������CO��H2ת��������CH3OCH3�������ӣ��ʴ�Ϊ��2CO��g��+4H2��g����CH3OCH3��g��+H2O��g����H����204.7kJ?mol-1���÷�Ӧ���������٣�ѹǿ����ʹƽ�����ƣ�CO��H2ת��������CH3OCH3�������ӣ�ѹǿ����ʹCO��H2Ũ�����ӣ���Ӧ��������
��3��ԭ����и���ʧȥ���ӣ���������ڸ�������������Ӧ���������Ϊ���ԣ�������ֱ��ȼ�ϵ�صĸ�����ӦΪ������ʧ��������̼��������ԭ���غ�͵���غ�д���缫��ӦΪ��CH3OCH3��16OH����12e����2CO32����11H2O�� ���ݸ����缫��Ӧʽ��֪һ�������ѷ��Ӿ����绯ѧ���������Բ���12�����ӵĵ�����
���㣺�����Ȼ�ѧ����ʽ��д����Ӧ�ȼ��㣻��������Է�Ӧ���ʺ�ƽ��״̬��Ӱ���Լ�ԭ���ԭ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����(H2NCONH2)��һ�ַdz���Ҫ�ĸߵ����ʣ��ڹ�ũҵ���������ŷdz���Ҫ�ĵ�λ��
��1����ҵ�Ϻϳ����صķ�Ӧ���£�
2NH3(l)+CO2(g) H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
H2O(l)+H2NCONH2(l) ��H=-103��7 kJ��mol-1
���д�ʩ��������������ص��������ʵ���
| A�����ø��� |
| B�����ø�ѹ |
| C��Ѱ�Ҹ���Ч�Ĵ��� |
| D����С��ϵ��CO2Ũ�� |
 H2NCOONH4(���������)(l) ��H1
H2NCOONH4(���������)(l) ��H1 H2O(l)+H2NCONH2(l) ��H2��
H2O(l)+H2NCONH2(l) ��H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���÷�Ӧԭ���о��������ȡ��⼰�仯����ķ�Ӧ����Ҫ���塣
��1���ڷ�Ӧ��2SO2(g)+O2(g) 2SO3(g)�Ļ����ϵ�У�SO3�İٷֺ������¶ȵĹ�ϵ����ͼ(�������κ�һ�㶼��ʾƽ��״̬����
2SO3(g)�Ļ����ϵ�У�SO3�İٷֺ������¶ȵĹ�ϵ����ͼ(�������κ�һ�㶼��ʾƽ��״̬����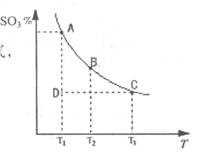
��2SO2(g)+O2(g) 2SO3(g)�ġ�H 0���>����<���������ں��¡���ѹʱ�����ƽ����ϵ��ͨ�뺤��ƽ�⽫ �ƶ�������������ҡ���������
2SO3(g)�ġ�H 0���>����<���������ں��¡���ѹʱ�����ƽ����ϵ��ͨ�뺤��ƽ�⽫ �ƶ�������������ҡ���������
�ڵ��¶�ΪT1����Ӧ���е�״̬Dʱ��V�� V�����>������<����=������
��2������ͼ��һ�������£�N2��H2�������淴Ӧ����1mol NH3�������仯ͼ���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽ ��(��H�ú�Q1��Q2�Ĵ���ʽ��ʾ��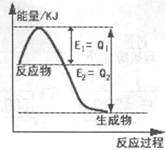
��25��Cʱ����a mol ? L�D1�İ�ˮ��b mol ? L�D1������������ϣ�������Һ��pH=7����c (NH4+) c(Cl�D)��a b���>������<����=������
��3����ˮ�к��д����Ի���̬��ʽ���ڵ��ȡ���Ԫ�ء���֪��250Cʱ��Ksp(AgCl)=1.6��10�D10mol2?L�D2��Ksp(AgI)=1.5��10�D16mol2?L�D2��
�� 250Cʱ���� 10mL0.002mol?L�D1�� NaCl��Һ�е��� 10mL0.002mol?L�D1AgNO3��Һ���а�ɫ�������ɣ���������Һ�м�������0.1mol ?L�D1��NaI��Һ����ɫ������ת��Ϊ��ɫ��������ԭ���� ���÷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�״���һ�ֳ��õ�ȼ�ϣ���ҵ�Ͽ�����CO��H2��һ�������ºϳɼ״���
��1����֪CO��g����H2��g����CH3OH��1����ȼ���ȡ�H�ֱ�Ϊ��-283.0kJ��mol��-285.8 kJ/mol��-726.5kJ/mol����CO�ϳɼ״����Ȼ�ѧ����ʽΪ�� ��
��2���ں����ܱ�������CO��H2������Ӧ���ɼ״���������Ũ���ڲ�ͬ�����µı仯״����ͼ��ʾ����ʼʱ������Ũ�����ߺ�8���Ӻ�״���Ũ������δ������4���Ӻ�8���Ӹı��������ͬ���� 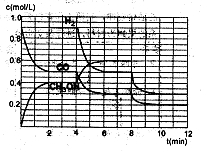
������˵����ȷ����
| A����ʼʱn��H2��Ϊ1��7mol |
| B����������ѹǿ�㶨ʱ��˵����Ӧ�ﵽƽ��״̬ |
| C��4����ʱ���ı�������������¶� |
| D��7����ʱ��v��CO��=v��CH3OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣�A��B��C��D��E��F����ѧ��ѧ�г��������ֶ�����Ԫ�أ��й�λ�ü���Ϣ���£�A������������Ӧ��ˮ���������⻯�ﷴӦ�������ӻ����C����һ�㱣����ú���У�F������������Ӧ��ˮ����������ᷴӦ������Ӧ��G�������ճ��������������Ľ������ױ���ʴ������ش��������⣺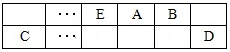
��1��AԪ�ص��⻯��ˮ��Һ��ʹ��̪����ԭ���õ��뷽��ʽ����Ϊ ��
��2��ͬ��ͬѹ�£���a L A�⻯��������b L D���⻯������ͨ��ˮ�У���������Һ��pH=7����a b(�>"��<����=��)
��3�������£���ͬŨ��F��G�����ӵ���Һ�еμ�NaOH��Һ��F��G��Ԫ���Ⱥ������F (OH)n��ȫ������pH��4.7��G (OH)n��ȫ������pH��2.8����ksp�ϴ���ǣ� ���ѧʽ��
��4��A��B�����������Ϊ7:16����ԭ�ӷ��ӣ��÷����ͷ��ڿ������仯ѧ���ÿ��������ĺ���У� ��
������ ������ЧӦ �۹⻯ѧ���� �ܳ������ƻ�
��5��A��C��ɵ�һ�����ӻ��������ˮ��Ӧ�������ּ�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��6����֪һ������E��������B2 (g)��ȼ�գ�����ܵIJ��P������ϵ������ͼ��ʾ����д��һ��������EB2(g) ��E��s����Ӧ����EB(g)���Ȼ�ѧ����ʽ ��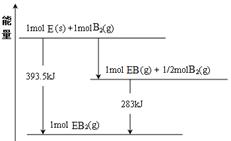
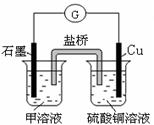
��7������D��G��ɵ�ij�ֻ��������Һ���У�����ͭƬ����Һ��������Ϊ��ɫ�����ݲ���������ķ�Ӧԭ��������Ƶ�ԭ���������ͼ��ʾ���䷴Ӧ��������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�Ҵ����ͺ�������35%,ʹȼ��ȼ�ո��ӳ��,ʹ�ó����Ҵ����ͣ�β���ŷŵ�CO
��̼�⻯����ƽ������30%����,��Ч�Ľ��ͺͼ������к���β���ŷš���������ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���NOx����Ч������Ϊ�����������Ҫ���⡣NOx��������У��γ����꣬��ɿ�����Ⱦ��NOx����һ�ֺ���ɫ���壬������ˮ�ķ���ʽ�� ��
��2����֪NO2��N2O4�Ľṹʽ�ֱ��� 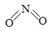 ��
��  ��
��
| ���� | NO2 | N2O4 | |
| ��ѧ�� | N��O | N��N | N��O |
| ���ܣ�kJ/mol�� | 466 | 167 | 438 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣���������Ȼ���еIJ���ѭ����ϵ���¡�
��1��H2S�ڿ����п���ȼ�ա�
��֪�� 2H2S(g) + O2(g)  2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
S(s) + O2(g)  SO2(g) ��H=��297.04 kJ/mol ��
SO2(g) ��H=��297.04 kJ/mol ��
H2S(g)��O2(g)��Ӧ����SO2(g)��H2O(g)���Ȼ�ѧ����ʽ�� ��
��2��SO2�Ǵ�����Ⱦ���ˮ�������õ�����SO2����������������¡�
�� SO2���ں�ˮ����H2SO3��H2SO3���ջ�����SO32��������뷽��ʽ�� ��
�� SO32�����Ա���ˮ�е��ܽ�������ΪSO42������ˮ��pH�� ������ߡ� �������䡱���͡�����
�� Ϊ������ˮ��pH���ɼ������ʵĺ�ˮ��ʹ���е�HCO3�����뷴Ӧ���䷴Ӧ�����ӷ���ʽ�� ��
�� ��������Ӧ��ͬʱ��Ҫ���������������ԭ���� ��
��3����Ȼ��ر���ԭ��ͭ�����ᆳ�������������ú���CuSO4��Һ���������������������ܵ�ZnS������ת��Ϊͭ����CuS�����û�ѧ�����ʾ��ZnSת��ΪCuS�Ĺ��̣� ��
��4��SO2��O2��H2SO4��Һ�п��Թ���ԭ��أ��为����Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��1��H2S��ȼ���Ȧ�H�� ��a kJ��mol��1����H2Sȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ ��
��2����֪�������£����ܱ���������H2��ԭWO2�ɵõ������١����¶ȹ���ʱ��WO2(s)��ת��ΪWO2 (g)����������·�Ӧ��
WO2 (s) + 2H2 (g)  W (s) + 2H2O (g)����H�� +66.0 kJ�� mol��1
W (s) + 2H2O (g)����H�� +66.0 kJ�� mol��1
WO2 (g) + 2H2 W (s) + 2H2O (g)����H �� ��137.9 kJ�� mol��1
W (s) + 2H2O (g)����H �� ��137.9 kJ�� mol��1
�����WO2 (s)  WO2 (g) �Ħ�H �� ______________________��
WO2 (g) �Ħ�H �� ______________________��
��3����ҵ�ϳ�������Ȼ��(��Ҫ�ɷ�ΪCH4)��CO2���и��������Ʊ�CO����Ӧ�Ļ�ѧ����ʽΪ��
CH4 + CO2 =" 2CO" + 2H2
��֪CH4��H2��CO��ȼ���ȷֱ�Ϊ890.3 kJ��mol-1��285.8 kJ��mol-1��283.0 kJ�� mol-1��������1 m3(��״��)CO��������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺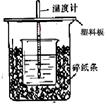
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ_____ ___��
��2���ձ���������ֽ����������___________________________��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ ���ƫ����ƫС�������ޡ�Ӱ�족����
��4��ʵ����60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� �����ȡ���������ȡ����������к��� �����ȡ���������ȡ������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com