
| ���� | �� | �� | �� | |
| ��ʼ��Ӧ��Ͷ���� | 2mol H2��l mol CO | 1mol CH3OH | 2mol CH3OH | |
| ƽ | c��CH��0H��/mol/L | C1 | C2 | c3 |
| �� | ��Ӧ�������仯ZkJ | x | y | z |
| �� | ��ϵѹǿ/Pa | P1 | P2 | P3 |
| �� | ��Ӧ��ת���� | a1 | a2 | a3 |
���� ��1���ٰ�ˮ��CO2��Ӧ����̼����泥��ݴ���д��
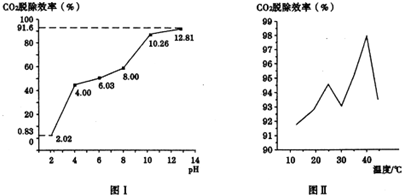
�ڸ���ͼ1������CO2�ѳ�Ч�������ռ���pH���������������ͨ�˰�ˮ������Ϊ0.052m3/h����״��������pHΪ12.81�İ�ˮ��������30min�����������CO2�ĺ���Ϊ12%�����CO2������
�۸����¶ȸ����ɵ�̼������ֽ����ɶ�����̼������
��2����A���ס�����Ƚϣ��Ѽ�ЧΪ��ʼ����1molCH3OH�����м״������ʵ���Ϊ��2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ���
B������Ŀ��֪����1molCH3OH�������仯Ϊ90.8kJ���ס���ƽ��״̬��ͬ����ƽ��ʱ�״�Ϊnmol�����㷴Ӧ����ֵ���ݴ��жϣ�
C���Ƚ��ҡ�����֪�����м״������ʵ���Ϊ�ҵ�2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ���
D���ס��Ҵ�����ͬ��ƽ��״̬�����1+��2=1����C�ķ�����֪��2����3���ݴ��жϣ�
����2H2��g��+CO��g��?CH3OH��g����ʼ��Ӧ��Ͷ����2mol H2��l mol CO���迪ʼʱ����ѹǿΪ2P��COѹǿΪP��������ʽ������ƽ�ⳣ��Kp=6.0��10-3��kPa��-2�������ƽ��ʱ����ѹ��������CH3OH�����ʵ�������Ϊ$\frac{��ѹ}{��ѹ}$���м��㣮
��� �⣺��1���ٰ�ˮ��CO2��Ӧ����̼����泥���Ӧ�ķ���ʽΪCO2+NH3•H2O�TNH4HCO3���ʴ�Ϊ��CO2+NH3•H2O�TNH4HCO3��
�ڸ���ͼ1������CO2�ѳ�Ч�������ռ���pH�����������Ϊ����ͨ�˰�ˮ������Ϊ0.052m3/h����״����������30minͨ��������к�������̼���Ϊ0.052m3/h��0.5h��12%=0.00312m3�������ʵ���Ϊ$\frac{0.00312��10{\;}^{3}L}{22.4L/mol}$��91.6%=0.13mol���ʴ�Ϊ��CO2�ѳ�Ч�������ռ���pH���������0.13mol��
����Ϊ�¶ȸ����ɵ�̼������ֽ����ɶ�����̼�����Դ�40�浽45���ѳ�CO2Ч�ʽ��ͣ��ʴ�Ϊ��̼����������ֽ����ɶ�����̼��
��2����A���ס�����Ƚϣ��Ѽ�ЧΪ��ʼ����1molCH3OH�����м״������ʵ���Ϊ��2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ�����2c1��c3����A����
B������Ŀ��֪����1molCH3OH�������仯Ϊ90.8kJ���ס���ƽ��״̬��ͬ����ƽ��ʱ�״�Ϊnmol�����ڼ�������a=90.8n������������b=90.8��1-n������a+b=90.8����B����
C���Ƚ��ҡ�����֪�����м״������ʵ���Ϊ�ҵ�2����ѹǿ�����ڷ�ӦCH3OH��g��?CO��g��+2H2��g����ƽ�������ɼ״��ķ����ƶ�����2p2��p3����C����
D���ס��Ҵ�����ͬ��ƽ��״̬�����1+��2=1����C�ķ�����֪��2����3������a1+a3��1����D��ȷ��
��ѡD��
���� 2H2��g��+CO��g��?CH3OH��g����ʼ��Ӧ��Ͷ����2mol H2��l mol CO���迪ʼʱ����ѹǿΪ2P��COѹǿΪP��
��ʼ��kPa�� 2P P 0
ת����kPa��48.0 24.0 24.0
ƽ�⣨kPa��2P-48 P-24 24
Kp=$\frac{24}{��2P-48��{\;}^{2}����P-24��}$=6.0��10-3��kPa��-2�����P=34.0kPa����ƽ����������CH3OH�����ʵ�������Ϊ$\frac{��ѹ}{��ѹ}$=$\frac{24}{30+24}$��100%=44.4%���ʴ�Ϊ��44.4%��
���� ���⿼�������̼������Ч�ʵ�ʵ��̽������Чƽ�������Լ�ƽ�ⳣ������ؼ���ȣ��ѶȽϴ�ע��ƽ��״̬�������Чƽ�������Ӧ�ã�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ά�ء���֬�������ʾ�����Ȼ�߷��ӻ����� | |
| B�� | ������ϩ������Ʒ������ʳƷ��װ | |
| C�� | ���ع��͡�����ʳ�ã�Ҳ���������Ʒ��� | |
| D�� | ������ʳ��ƾ����˵��ۡ������ǡ��Ҵ��Ļ�ѧ�仯���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | Fe2+ | Fe3+ | Cu2+ |
| ��ʼ����ʱ��pH����ʼŨ��Ϊ1.0mol/L�� | 6.5 | 1.5 | 4.2 |
| ������ȫʱ��pH | 9.7 | 3.2 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�11.2LSO3������ԭ����Ϊ1.5NA | |
| B�� | ���³�ѹ�£�1.8g H2O�к��еĵ�����Ϊ0.8NA | |
| C�� | ���³�ѹ�£�48g O2��O3�Ļ�����к��е���ԭ����Ϊ3NA | |
| D�� | ��״���£�0.1mol Cl2������NaOH��Һ��Ӧʱ��ת�Ƶĵ�����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ͱ��� | B�� | �����2��2-�������� | ||
| C�� | �Ҵ����Ҷ��� | D�� | 1��1-���������1��2-�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �縺�ԣ�X��Y | |
| B�� | ���ڱ��У�X������Y���ұ� | |
| C�� | ��X��Y�γɻ������X�����ۣ�Y�Ը��� | |
| D�� | ��̬�⻯����ȶ��ԣ�HmYǿ��HnX |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KBr | B�� | N2 | C�� | HBr | D�� | NaOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com