
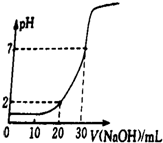
 �����£�
�����£����� ��1������PH=-lg��c��H+������PH�����c��H+����c��OH-��=10-14������ˮ�������c��H+����
��2������n��H+��=n��OH-�����㣻
��3���������ᡢ����������Һ�����ʵ���Ũ�ȷֱ�Ϊc��HCl����c��NaOH������NaOH��Һ���Ϊ20mL�����ù�ϵʽHCl��NaOH���ͼ���֪���������c��H+��=$\frac{10c��HCl��-20c��NaOH��}{30}$=0.01mol/L����NaOH��Һ���Ϊ30mL�����ù�ϵʽHCl��NaOH�����ͼ���֪����Һ�����ԣ�ǡ�÷�Ӧ����10c��HCl��=30c��NaOH����������������㣻
�ڸ���n=cV�ֱ������Ȼ��⡢�������Ƶ����ʵ�����Ȼ���������Һ��������Ũ�ȣ�����������Һ��pH��
�۸����к͵ζ�����ȷ�IJ����������
��� �⣺��1��PH=-lg��c��H+��=-lg��0.01��=2����c��H+����c��OH-��=10-14��֪��c��OH-��=$\frac{1{0}^{-14}}{0.01}$mol/L=1��10-12mol/L��
�ʴ�Ϊ��2�� 1��10-12��
��2��pH=13��Ba��OH��2��Һ a L��pH=3��H2SO4��Һb L��ϣ������û����Һ�����ԣ�����n��H+��=n��OH-����
����0.1mol/L��2��aL=0.001mol/L��2��bL��
��a��b=01��100��
�ʴ�Ϊ��1��100��
��3��������ᡢNaOH��Һ�����ʵ���Ũ�ȷֱ�Ϊc��HCl����c��NaOH����
��NaOH��Һ���Ϊ20mL�����ͼ���֪�����������c��H+��=$\frac{10c��HCl��-20c��NaOH��}{30}$�T0.01mol/L��
��NaOH��Һ���Ϊ30mL�����ͼ���֪����Һ�����ԣ�ǡ�÷�Ӧ������10C��HCl��=30c��NaOH����
����ʽ������ã�c��HCl��=0.09mol/L��c��NaOH��=0.03mol/L��
�ٸ��ݼ����֪���������Ũ��Ϊ0.09mol/L��
�ʴ�Ϊ��0.09mol/L��
�ڵ��μ�NaOH��Һ��26mLʱ������ͼ���֪�����Һһ��Ϊ���ԣ�����Һ��������Ũ��Ϊ��$\frac{0.09mol/L��0.01L-0.03mol/L��0.026L}{0.01L+0.026L}$=$\frac{1}{3}$��10-2mol/L�����Һ��pH=-lg$\frac{1}{3}$��10-2=2+lg3��
�ʴ�Ϊ��2+lg3��
�۴ӵζ�������pH�仯��֪���к͵ζ����ٶ�ֻ�����ȿ���������ܳ��������������һ�������pH�仯�ܴ�
�ʴ�Ϊ���ȿ���������ܳ�������
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㣬��Ŀ�Ѷ��еȣ���ȷ��Һ���������ҺpH�Ĺ�ϵΪ���ؼ���ע��������ҺpH�ı���ʽ�����㷽����������ؿ���ѧ���ķ�����������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�100g46%�Ҵ�ˮ��Һ�к���Hԭ����Ϊ12 NA | |
| B�� | 23gNa������O2��Ӧ������Na2O ��Na2O2�Ļ���ת�Ƶĵ�����Ϊ NA | |
| C�� | ��״���£�2.24 LCCl4���еĹ��ۼ���Ϊ0.4 NA | |
| D�� | 1 molN2��4molH2��Ӧ���ɵ�NH3������С��2 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe��ϡHNO3��ϡH2SO4��Ӧ�������ݲ�����˵��Fe��������������û���Ӧ | |
| B�� | ������Fe��Cl2��ȼ������FeCl2��FeCl3 | |
| C�� | �Ⱥ����ʵ�����Fe2O3��Fe�ֱ�����ͬһϡ����ʱ������ų� | |
| D�� | Fe2O3$\stackrel{HCl��aq��}{��}$FeCl3��aq��$\stackrel{��}{��}$��ˮFeCl3���ڸ�������������ʵ��ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ3��4 | |
| B�� | �÷�Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ1��4 | |
| C�� | N��������������Ǽ��Է��� | |
| D�� | M�ǻ�ԭ����仹ԭ�Ա�NH3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����һ�����ϣ�һ�ֺϳ�·�����£�
����һ�����ϣ�һ�ֺϳ�·�����£�
 ��
�� ��
�� ����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��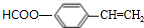 ��
�� ��д�ṹ��ʽ��
��д�ṹ��ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����
�����
 ��b��
��b�� ��c��H2O2��d��
��c��H2O2��d�� ��e��
��e�� ��f��
��f�� ��g��O3��h��
��g��O3��h��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
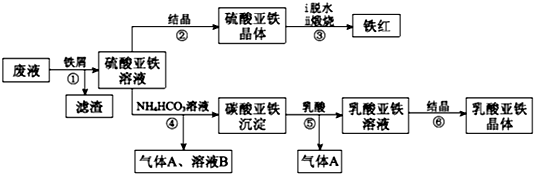
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 12C��14C | B�� | CH3CH2OH��CH3COOH | ||
| C�� | ��������� | D�� | CH3CH2CH2CH3��CH3CH��CH3��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com