
��1��ijѧ����������ƽ����һ��С�ձ�����������С�ձ�������Ϊ32.6g���á�������ʾ�������Ϸ����룬��������ʾ��������ȡ���룬���ü�ͷ���±�����գ���ʾ�������̣�����ͼ3�D2��ʾ��������ϻ��������λ�ã�����|����ʾ����
��������/g | 50 | 20 | 20 | 10 | 5 |
ȡ��������� |

��2��ͼ3�D3��ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1mL������̶�AΪ4����Ͳ�е�Һ��������__________mL��
��3������![]() ��������Һ200mL����IJ�������Ϊ___________��
��������Һ200mL����IJ�������Ϊ___________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ�Ž�һ��2011��2012ѧ���һ��ѧ�����п��Ի�ѧ���� ���ͣ�058
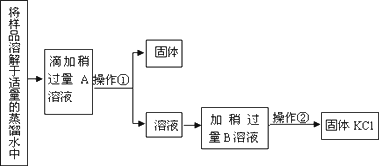
ʵ��������һƿ�Ȼ��غ��Ȼ��ƵĹ������ͨ�������ʵ���ȥ�Ȼ������Ȼ��ƣ������ô������Ȼ�������һ��Ũ�ȵ��Ȼ�����Һ������ʵ�鲽����д���пհף�
�ش���������
(1)�����A��________��(�����ʽ����ͬ)
(2)�����ڵ�������________�������١��ھ�Ҫ�õ��IJ���������________��
(3)B������________�����������B��Ӧ�����ӷ���ʽΪ________��
(4)��ȡһ����������KCl������һ��Ũ�ȵ��Ȼ�����Һ��
(��)ijͬѧ������������ȡһ��������KCl���Ʒ�����ͼ��ʾ����ƽ��ָ���ڱ���м䣮�˲����Ƿ���ȷ________(����ȷ����ȷ)�������˲�������������KCl��ʵ������Ϊ________g (��֪�ձ�������Ϊ12.9��)��

(��)ijѧ����ȷ������KCl��������ձ��У�����Լ30��������ˮ���ò���������ʹ���ܽ⣮����Һ���ձ�����100��������ƿ�У�Ȼ��������ƿ��С�ĵؼ�����ˮ��ֱ��Һ��ӽ��̶�1��2���״������ý�ͷ�ιܼ�����ˮ��ʹ��Һ������͵�ǡ����̶����У�������ƿ�ǽ�������ҡ�ȣ����������еĴ�����________��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
��1����������ƽ��С�ձ��Ƴ�һҩ����Ʒ������������ȷ������˳��Ϊ____________��
�ٵ���㣬������ƽ���ҵ�С��˿��ʹָ�뾲ֹʱ�պô��ڱ�߿̶����㴦�ڽ����벦���ڿ̶ȳ�������㴦�۳����յ�С�ձ���ȡ��Ʒ����С�ձ����ٳ����ݽ�����Ż������
��2���ܽ���Ʒ����������____________��
��3�������A��Һ��____________���ӹ����Լ���Ŀ����____________��ȷ��B�����ķ�����____________��
��4������ʱ��ijѧ��������ͼ��ʾ�IJ�����������У���____________����____________��
��5��������ϴ�ӵ�ԭ����______________��ϴ�ӷ���Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������һƿ���������Ѳ��ֱ��ʣ�����ˮ�ݣ�����ΪNa2CO3����ijѧ�����������ʵ����ȷ���䴿�ȣ�������ͼʵ�鲽����д�йؿհ״���
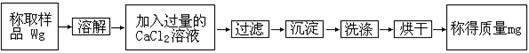
��1����������ƽ��С�ձ��Ƴ�һ����Ʒ������������ȷ�IJ���˳��Ϊ _________________________________________________
�ٵ���㣬������ƽ���ҵ�С��˿��ʹָ�뾲ֹʱ�պô��ڱ�߿̶����㴦
�ڽ����벦���ڿ̶ȳ�������㴦 �۳����յ�С�ձ�
��ȡ��Ʒ����С�ձ����ٳ��� �ݽ�����Ż������
��2���ܽ���Ʒ����Ҫ������_______________________��
��3�������![]() ��Һ������Ŀ����________________________________________
��Һ������Ŀ����________________________________________
ȷ�Ϲ����ķ�����________________________________________________________��
��4��ϴ�ӵij���Ŀ���ǣ�________________________________��
ϴ�ӷ�����_______________________________________________________________��
��5������CaCO3��Է�������Ϊ100������Ʒ��NaOH������������______________��(3��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����13�֣���һ��ʵ������������������1.00mol/L������������Һ0.2L���ش���������
��1�������й�ʹ��������ƽ����������ȷ���� ��
A������ǰ�ȵ�����ƽ����㡣
B������ʱ���̷ű���������̷����롣
C���������Ʊ�����ڲ��������������
D������ʱ��Ӧ�ȼ�����С������������������ƽƽ�⡣
E��������ϣ�Ӧ����ƽ�Ż�������С�
��2��ijͬѧ��������ƽ��ȡһʢ����������ҩƷ���ձ����������Ϸ���30g���룬����λ������ͼ��ʾ����ƽ��ָ��ָ�ڱ�ߵ��м䡣���ձ��ͷۼ���������Ϊ_________�ˡ�
��������98%��ŨH2SO4����=1.84g/cm3�����Ƴ�0.5mol/L��ϡH2SO4 500ml�IJ����������£��ټ�������Ũ�������� ����ȡһ�������Ũ���� ���ܽ� ��ת�ơ�ϴ�� �ݶ��ݡ�ҡ�� ���밴Ҫ����գ�
��1�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ�� mL��Ͳ��á�
��2���ڢݲ�ʵ��IJ����ǣ�����������_______________________________________________
��3��ijѧ�������в�����ʹŨ��ƫ�͵�ԭ���ǣ� ��
�� ����Ͳ��ȡŨ����ʱ�����Ӷ�����
�� ��Ͳ��Ũ����ȫ��ת���ձ���ϡ�ͺ���ת�Ƶ�100ml����ƿ�У��ձ�δϴ�ӡ�
�� �ò���������������Һת�Ƶ�����ƿ������Һ����������ƿ���档
�� Ũ������С�ձ���ϡ�ͺ�û����ȴ������ת��������ƿ��
�� �ý�ͷ�ιܼ�����ˮʱ����������ʹҺ�泬���˿̶��ߣ������õι���ȥ�����ˮ��ʹ��Һ��ʯ�պ���̶������С�
�� �μ�������ˮ��ʹ��Һ����պ���̶������У�����ƿ������ҡ�Ⱥ��ã�����Һʯ�ȿ̶��ߵͣ��ټ�ˮ���̶��ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������һƿ���������Ѳ��ֱ��ʣ�����ˮ�ݣ�����ΪNa2CO3����ijѧ�����������ʵ����ȷ���䴿�ȣ�������ͼʵ�鲽����д�йؿհ״���

��1����������ƽ��С�ձ��Ƴ�һ����Ʒ������������ȷ�IJ���˳��Ϊ _________________________________________________
�ٵ���㣬������ƽ���ҵ�С��˿��ʹָ�뾲ֹʱ�պô��ڱ�߿̶����㴦
�ڽ����벦���ڿ̶ȳ�������㴦 �۳����յ�С�ձ�
��ȡ��Ʒ����С�ձ����ٳ��� �ݽ�����Ż������
��2���ܽ���Ʒ����Ҫ������_______________________��
��3�������CaCl2��Һ������Ŀ����________________________________________
ȷ�Ϲ����ķ�����_____________________________________________________��
��4��ϴ�ӵij���Ŀ���ǣ�________________________________��ϴ�ӷ�����_______________________________________________________________��
��5������CaCO3��Է�������Ϊ100������Ʒ��NaOH������������_________����3�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com