
 ���ֶ�����Ԫ��X��Y��Z��W��Q��ԭ��������������X��Y�Ƿǽ���Ԫ��X��Y��QԪ�ص�ԭ������ܼ��ϵ�������ȣ�ZԪ��ԭ�ӵ������������Ǵ�����������WԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ���ԭ����p�Dz���s�Dz����������ȣ�QԪ�ص����ֱܷ���I1=496��I2=4562��I3=6912���ش��������⣺
���ֶ�����Ԫ��X��Y��Z��W��Q��ԭ��������������X��Y�Ƿǽ���Ԫ��X��Y��QԪ�ص�ԭ������ܼ��ϵ�������ȣ�ZԪ��ԭ�ӵ������������Ǵ�����������WԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ���ԭ����p�Dz���s�Dz����������ȣ�QԪ�ص����ֱܷ���I1=496��I2=4562��I3=6912���ش��������⣺���� ZԪ��ԭ�ӵ������������Ǵ�����������������������8�����ӣ���Z����2�����Ӳ㣬����㺬��4�����ӣ�ΪCԪ�أ�WԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ���ԭ����p�Dz���s�Dz����������ȣ���Wԭ�Ӻ���2��s�Dz��1��p�Dz㣬�ܹ�����4�����ӣ�ΪOԪ�أ�Q�ĵڶ������ܾ�������Qԭ���������1�����ӣ����ԭ��������֪QΪNa��X��Y�Ƿǽ���Ԫ�أ�X��Y��QԪ�ص�ԭ������ܼ��ϵ�������ȣ�������ܼ��Ϻ���1�����ӣ���XΪH��YΪBeԪ�أ��ݴ˽��н��
��� �⣺ZԪ��ԭ�ӵ������������Ǵ�����������������������8�����ӣ���Z����2�����Ӳ㣬����㺬��4�����ӣ�ΪCԪ�أ�WԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ���ԭ����p�Dz���s�Dz����������ȣ���Wԭ�Ӻ���2��s�Dz��1��p�Dz㣬�ܹ�����4�����ӣ�ΪOԪ�أ�Q�ĵڶ������ܾ�������Qԭ���������1�����ӣ����ԭ��������֪QΪNa��X��Y�Ƿǽ���Ԫ�أ�X��Y��QԪ�ص�ԭ������ܼ��ϵ�������ȣ�������ܼ��Ϻ���1�����ӣ���XΪH��YΪBeԪ�أ�
��l��QΪNaԪ�أ�Na��ԭ������Ϊ11��������Ų�Ϊ��1s22s22p63s1��
�ʴ�Ϊ��1s22s22p63s1��
��2��Q��W�γɵĻ�����Q2W2ΪNa2O2����������Ϊ���ӻ������ѧʽ�к������Ӽ���O-O���Թ��ۼ���
�ʴ�Ϊ�����Ӽ����Ǽ��Թ��ۼ���
��3��YΪBeԪ�أ�Be���Ԫ���γɵĻ�����ΪBeF3��BF3��B�ļ۵��ӽṹΪ2s22p1���γɷ���ʱ������sp2�ӻ�������sp2�ӻ�����ֱ�������Fԭ�ӵ�p����ɼ�����BF3����Ϊƽ�������Σ����ڷǼ��Է��ӣ�
XΪH��YΪBe��Y��X���γɾ�������ṹ�Ļ�����Be2H6���ýṹ��Be�γ�4�����ۼ�������sp3�ӻ���
�ʴ�Ϊ��ƽ�������Σ��Ǽ��ԣ�sp3��
��4��Y��OH��3ΪB��OH��3��B��OH��3һԪ���ᣬ����Beԭ����ȱ���Ӷ����γ���λ������Y��OH��3��ˮ��Һ�еĵ��뷽��ʽΪ��B��OH��3+H2O?[B��OH��4]-+H+��
�ʴ�Ϊ��B��OH��3+H2O?[B��OH��4]-+H+��
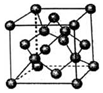
��5����ZΪCԪ�أ�����ͼʾ��֪���þ�������ԭ�Ӿ��壬
�ʴ�Ϊ��ԭ�Ӿ��壻
�ڸ���ͼ֪��Cԭ����λ����4��ÿ��̼ԭ�����ӹ��ۼ�����Ϊ��$\frac{1}{2}$��4=2�����Ժ�1mol Zԭ�ӵĸþ����й���2mol��ѧ����
�ʴ�Ϊ��2��
��Cԭ�Ӱ뾶Ϊrpm������Ӳ��Ӵ�ģ�Ϳ�֪����Խ����ķ�֮һ����ԭ���붥���ϵ�ԭ�ӽ������辧���߳�Ϊa������$\frac{1}{4}$����$\sqrt{3}$a��=2r����a=$\frac{8}{\sqrt{3}}$rpm���þ������Ϊ����$\frac{8}{\sqrt{3}}$r��3pm3��
�þ�����Cԭ�Ӹ���=4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8��1�������е�����Ϊ��$\frac{8M}{{N}_{A}}$g��
���Ըþ�����ܶ�Ϊ����=$\frac{m}{V}$=$\frac{\frac{8M}{{N}_{A}}}{��\frac{8r}{\sqrt{3}}��^{3}p{m}^{3}}$=$\frac{\frac{8M}{{N}_{A}}}{��\frac{8r}{\sqrt{3}}��1{0}^{-10}��^{3}}$g•cm-3=$\frac{3\sqrt{3}M}{64{N}_{A}{r}^{3}}$��1030g•cm-3��
�ʴ�Ϊ��$\frac{3\sqrt{3}M}{64{N}_{A}{r}^{3}}$��1030��
���� ���⿼���Ϊ�ۺϣ��漰�������㡢Ԫ���ƶϡ�ԭ�ӽṹ��Ԫ�������ɵ�Ӧ�á�ԭ���ӻ���ʽ�����͡����ӷ���ʽ��д�����ۼ����͵�֪ʶ����Ŀ�ѶȽϴ��ƶ�Ԫ��Ϊ���ؼ���ע�����վ�̯���ھ��������е�Ӧ�ã�����������ѧ���ķ������������Ӧ��������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ����Դ�� | B�� | ��ȼ�գ��ų������� | ||
| C�� | ��������Ⱦ | D�� | ��ȡH2������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
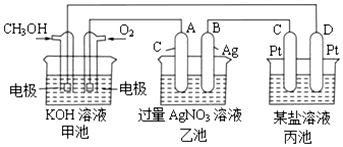
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ����˴Ź�����������2���źţ��μ�ͼ1����
����˴Ź�����������2���źţ��μ�ͼ1����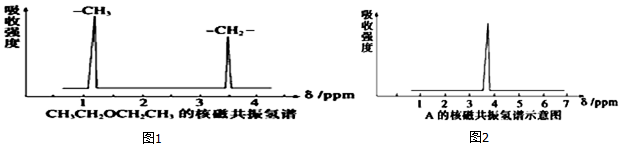
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
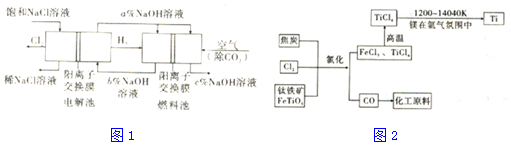
| ���� | Na2CO3 | NaHCO3 | NaClO | NaHSO3 |
| pH | 11.6 | 9.7 | 10.3 | 5.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
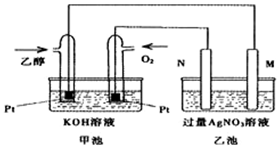 ��֪�״����Ҵ�������Ҫ���л�����ԭ�ϣ��ش��������⣺
��֪�״����Ҵ�������Ҫ���л�����ԭ�ϣ��ش��������⣺| ��ѧ��Ӧ��ƽ�ⳣ�� | ƽ�ⳣ����ֵ | ||
| 500�� | 800�� | ||
| ��2H2��g��+CO��g��?CH3OH��g�� | K1 | 2.5 | 0.15 |
| ��H2��g��+CO2��g��?H2O��g��+CO��g�� | K2 | 1.0 | 2.50 |
| ��3H2��g��+CO2��g��?CH3OH��g��+H2O��g�� | K3 | 2.5 | 0.375 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����������Ԫ��X��Y��Z��W��ԭ������������������Ԫ���γɵĵ�������Ϊm��n��p��q�� r��t��u����ЩԪ����ɵĶ�Ԫ���������uΪ���Ѿ����־��ɷ֣�25��ʱ��0.01mol/L��v��Һ�У�$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1.0��10-10���������ʵ�ת����ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
����������Ԫ��X��Y��Z��W��ԭ������������������Ԫ���γɵĵ�������Ϊm��n��p��q�� r��t��u����ЩԪ����ɵĶ�Ԫ���������uΪ���Ѿ����־��ɷ֣�25��ʱ��0.01mol/L��v��Һ�У�$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1.0��10-10���������ʵ�ת����ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ԭ�Ӱ뾶�Ĵ�С��X��Y��Z��W | |
| B�� | Ԫ�صķǽ����ԣ�Y��W��X | |
| C�� | t��ֻ�����Ӽ� | |
| D�� | v������ˮ�ĵ����u�ܴٽ�ˮ�ĵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H+��AlO2-��CO32- | B�� | K+��Ba2+��SO42- | C�� | NH4+��OH-��NO3- | D�� | Fe3+��NO3-��Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com