
| Ԫ�� | A | B | C | D |
| ���� �ṹ ��Ϣ | ��̬ԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��� | ��̬ԭ�ӵ�M����1�ԳɶԵ�p���� | ��̬ԭ�Ӻ�������Ų�Ϊ[Ar]3d104sx����+1��+2���ֳ������ϼ� | �����ֳ��������������һ����ұ��ҵ���õĻ�ԭ�� |
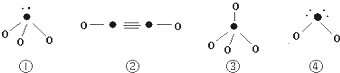
���� Aԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��ӣ���Χ�����Ų�Ϊ2s22p3����AΪNԪ�أ�Bԭ�ӵ�M����1�ԳɶԵ�p���ӣ���Χ�����Ų�Ϊ3s23p4����BΪSԪ�أ�Cԭ�ӵĺ�������Ų�Ϊ[Ar]3d104sx����+1��+2���ֳ������ϼۣ���CΪCuԪ�أ�D�����ֳ��������������һ����ұ��ҵ���õĻ�ԭ������DΪCԪ�أ��ݴ˽��
��� �⣺Aԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��ӣ���Χ�����Ų�Ϊ2s22p3����AΪNԪ�أ�Bԭ�ӵ�M����1�ԳɶԵ�p���ӣ���Χ�����Ų�Ϊ3s23p4����BΪSԪ�أ�Cԭ�ӵĺ�������Ų�Ϊ[Ar]3d104sx����+1��+2���ֳ������ϼۣ���CΪCuԪ�أ�D�����ֳ��������������һ����ұ��ҵ���õĻ�ԭ������DΪCԪ��
��1��CΪCuԪ�أ���̬�����Ų�ʽΪ��[Ar]3d104s1��DΪ̼Ԫ�أ���������Ų�ʽΪ1s22s22p2���ɶԵ�������δ�ɶԵ�����֮��Ϊ4��2=2��1��
�ʴ�Ϊ��[Ar]3d104s1��2��1��
��2��NԪ�صĵ縺�Ժ�ǿ����������֮�����������ʰ����ķе��ͬ��������Ԫ���⻯��е�ߣ�
�ʴ�Ϊ���ߣ���������֮����������
��3��DԪ�����������ΪCO2��ͬ��������Ԫ�����������ΪSiO2��CO2Ϊ���Ӿ��壬SiO2��ԭ�Ӿ��壬�����۵�CO2��SiO2���ʴ�Ϊ���ͣ�CO2Ϊ���Ӿ��壬SiO2��ԭ�Ӿ��壻
��4��CԪ�ص�������ΪCuSO4��AԪ�ص��⻯��ˮ��ҺΪ��ˮ����CuSO4��Һ����μ��������ˮ�����ɵ������Ļ�ѧʽΪ[Cu��NH3��4]SO4����������Cu2+����λ��NH3֮������λ�����ϣ��ڽ�������[Cu��NH3��4]2+���������SO42-֮�������Ӽ����ϣ�����������������к��й��ۼ���
�ʴ�Ϊ��[Cu��NH3��4]SO4��ABD��
��5���٣�����ԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ���Ϊ�����Σ�����������������IJ��غϣ����ڼ��Է��ӣ�
�ڣ�����ԭ�ӳ�2��������1��������û�жԹ¶Ե��ӣ��ӻ������Ϊ3����ȡsp2�ӻ���Ϊֱ���ͽṹ��������������������غϣ����ڷǼ��Է��ӣ�
�ۣ�����ԭ�ӳ�4��������û�жԹ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ���Ϊ��������ṹ��������������������غϣ����ڷǼ��Է��ӣ�
�ܣ�����ԭ�ӳ�2������������2�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ���ΪV�ν�ϣ�����������������IJ��غϣ����ڼ��Է��ӣ�
�ڢڵķ�������2��������1������������Ϊ�Ҽ�����������1���Ҽ���2���м����ʷ����к���3���Ҽ���2���м���
�ʴ�Ϊ���٢ۢܣ��٢ܣ�3��2��
���� ���⿼��ṹ��λ�ù�ϵ����������Ų����ɡ���ѧ��������ṹ���������ʡ��ӻ����۵ȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ�ּ�ڿ���ѧ���Ի���֪ʶ���������գ�


 ��������������������ϵ�д�
��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
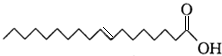 �������жϲ���ȷ���ǣ�������
�������жϲ���ȷ���ǣ�������| A�� | ���������ϩ�ụΪͬϵ�� | |
| B�� | �����ʵķ���ʽΪC18H34O2 | |
| C�� | ������������ˮ���ڿ����в��ױ��� | |
| D�� | �����ʵ�̼�����о���νṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
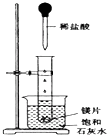 ��ͼ��ʾ�����Թܷ���ʢ��25��ı���ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У��Իش����лش�
��ͼ��ʾ�����Թܷ���ʢ��25��ı���ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5mL�������Թ��У��Իش����лش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
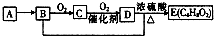 ��֪���л�������Aֻ��C��H����Ԫ���������ʹ��ˮ��ɫ�������������������һ������ʯ�ͻ�����չˮƽ��A��B��C��D��E����ͼת����ϵ�������ƶϴ�����ǣ�������
��֪���л�������Aֻ��C��H����Ԫ���������ʹ��ˮ��ɫ�������������������һ������ʯ�ͻ�����չˮƽ��A��B��C��D��E����ͼת����ϵ�������ƶϴ�����ǣ�������| A�� | ����A�ͼ����ѡ��ʹ�����Ը��������Һ | |
| B�� | D�к����Ȼ�������D���ʿ������ˮ���е�ˮ�� | |
| C�� | ͼ���漰�ķ�Ӧ�����мӳɡ�ȡ�������� | |
| D�� | B+D��E�Ļ�ѧ����ʽ��C2H5OH+CH3COOH��CH3COOC2H5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
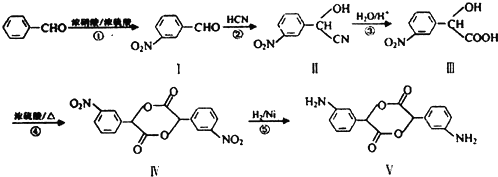
��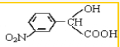 ��
��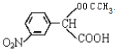 +H2O����Ҫ��д����Ӧ���������ñ�ͪ��
+H2O����Ҫ��д����Ӧ���������ñ�ͪ�� 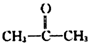 �����滯����I������Ӧ�ڡ���Ӧ�ۿ��Եõ���������������л��������м��壩����������Ľṹ��ʽΪCH3CHOH��CH3��COOH��
�����滯����I������Ӧ�ڡ���Ӧ�ۿ��Եõ���������������л��������м��壩����������Ľṹ��ʽΪCH3CHOH��CH3��COOH��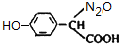 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
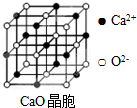 ��ҵ�ϣ����������з�Ӧ�ϳ������谷��
��ҵ�ϣ����������з�Ӧ�ϳ������谷��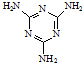 �����е�ԭ�ӵ��ӻ���ʽ��sp2��sp3��
�����е�ԭ�ӵ��ӻ���ʽ��sp2��sp3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��AgNO3��CuCl2�Ļ����Һ�л���ͨ�백ˮ��Һ | |
| B�� |  ��NaOH��Ca��OH��2�Ļ����Һ��ͨ��CO2 | |
| C�� | 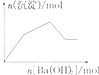 KAl��SO4��2��Һ����μ���Ba��OH��2��Һ | |
| D�� | 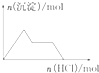 NaAlO2��Һ����μ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
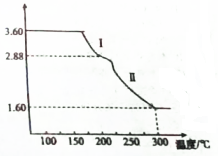 �ȼ��仯���������������������Ź㷺����;���ش��������⣺
�ȼ��仯���������������������Ź㷺����;���ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com