
��12�֣���1��H+����H2O�γ�H3O+��H3O+��Oԭ�Ӳ���  �ӻ�������DZ�ˮ���� �����С������
�ӻ�������DZ�ˮ���� �����С������
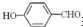
��2���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ʾ��ͼ��δ��˳�������������־����о���������ͬ���� ������д��ţ���
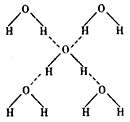
��3���ڱ��ľ����У�ÿ��ˮ���������ڵ� 4 ��ˮ�����γ��������ͼ��ʾ������֪���������ȣ��� 1 mol ˮ�ɱ�ת��Ϊˮ����������������51KJ/mol��������⣬ˮ���Ӽ仹���ڷ��»�����11 kJ / mol �� , �������������ġ����ܡ����ƻ� lmol ��������������Ϊ ����ġ����ܡ����� kJ / mol ��
����ġ����ܡ����� kJ / mol ��
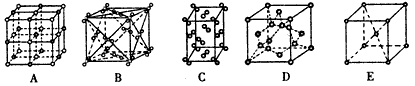
��4��CaO��NaCl�ľ���ͬΪ���������ṹ����֪CaO�����ܶ�Ϊag��cm-3��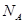 ��ʾ�����ӵ���������CaO�������Ϊ cm3��
��ʾ�����ӵ���������CaO�������Ϊ cm3��
��5����������Һ��,������������ԭ��Ӧ�б������뱻��ԭ�ĵ�Ԫ�ص����ʵ���֮��Ϊ2:5,
��KBiO3+��MnSO4+��H2SO4==��Bi2(SO4)3+�� +�� +��
д��Biͬ�����������Ԫ�صĵ����Ų�ʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��
�е��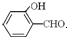 �ߣ�ԭ����
�ߣ�ԭ����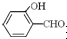 �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ����������� �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������| 224 |
| aNA |
| 224 |
| aNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
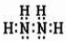
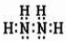
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0117 ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com