
��ѧ���õ���ɫ��pH��ֽ�ⶨ��Һ������ԡ���25��ʱ,����Һ��pH=7,��ֽ����ɫ;��pH< 7 ,��ֽ���ɫ;��pH > 7,��ֽ����ɫ��
����֪ˮ�д�������ƽ��: H2O + H2O H3O+ +OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
A.��ˮ�м���NaHSO4 B.��ˮ�м���Cu(NO3)2 C.������100�� D.��ˮ�м���(NH4)2SO4
����Ҫ��pH��ֽ�ⶨ100���ˮ��pH��������ֽ�� ɫ,��Һ�� ��(���ᡢ�����)��
�Ǵ�����ˮ��Һ�Լ��Զ��������������ӷ���ʽ��ʾ�Լ��Ե�ԭ��
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ���õ���ɫ��pH��ֽ�ⶨ��Һ������ԡ���25��ʱ,����Һ��pH=7,��ֽ����ɫ;��pH< 7 ,��ֽ���ɫ;��pH > 7,��ֽ����ɫ��
����֪ˮ�д�������ƽ��: H2O + H2O ![]() H3O+ + OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
H3O+ + OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
A.��ˮ�м���NaHSO4 B.��ˮ�м���Cu(NO3)2 C.������100�� D.��ˮ�м���(NH4)2SO4
����Ҫ��pH��ֽ�ⶨ100���ˮ��pH��������ֽ�� ɫ,��Һ�� ��(���ᡢ�����)��
�Ǵ�����ˮ��Һ�Լ��Զ��������������ӷ���ʽ��ʾ�Լ��Ե�ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ�ɶ���ʵ����ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��ѧ���õ���ɫ��pH��ֽ�ⶨ��Һ������ԡ���25��ʱ,����Һ��pH=7,��ֽ����ɫ;��pH< 7 ,��ֽ���ɫ;��pH > 7,��ֽ����ɫ��
����֪ˮ�д�������ƽ��: H2O + H2O 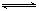 H3O+ + OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
H3O+ + OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
| A����ˮ�м���NaHSO4 | B����ˮ�м���Cu(NO3)2 | C��������100�� | D����ˮ�м���(NH4)2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���Ĵ�ʡ�ɶ��и߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��ѧ���õ���ɫ��pH��ֽ�ⶨ��Һ������ԡ���25��ʱ,����Һ��pH=7,��ֽ����ɫ;��pH< 7 ,��ֽ���ɫ;��pH > 7,��ֽ����ɫ��
����֪ˮ�д�������ƽ��: H2O + H2O  H3O+ +
OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
H3O+ +
OH-����Ҫʹƽ�������ƶ�,�����õ���Һ������,ѡ��ķ�����
A.��ˮ�м���NaHSO4 B.��ˮ�м���Cu(NO3)2 C.������100�� D.��ˮ�м���(NH4)2SO4
����Ҫ��pH��ֽ�ⶨ100���ˮ��pH��������ֽ�� ɫ,��Һ�� ��(���ᡢ�����)��
�Ǵ�����ˮ��Һ�Լ��Զ��������������ӷ���ʽ��ʾ�Լ��Ե�ԭ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com