
��ѧ�ڻ�������������ʮ����Ҫ�����ã��绯ѧ���ⷨ����������ˮ�������ε���Ⱦ���绯ѧ����NO3����ԭ��ͼ��ʾ������˵������ȷ���ǣ�
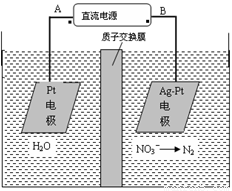
A��AΪ��Դ����
B��������ӦʽΪ��2H2O��4e��=4H����O2��
C������������ת����2mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ10.4��
D��������ӦʽΪ��2NO3����6H2O��10e����N2����12OH��
C
��������
������������⿼����ԭ����Ӧ�ã����÷�Ӧ���Ͷ����������ԭ��ͼ֪��Ag-Pt�缫��NO3-������ԭ��Ӧ�����Ag-Pt�缫Ϊ�������缫��ӦʽΪ��2NO3����6H2O��10e����N2����12OH������BΪ������AΪ��Դ������Pt�缫Ϊ���ص��������缫��ӦʽΪ��2H2O��4e��=4H����O2����A���������ԭ��ͼ֪����Ag-Pt�缫��NO3-������ԭ��Ӧ�����Ag-Pt�缫Ϊ��������BΪ������AΪ��Դ��������ȷ��B������Ϊ��Һ�е��������ŵ磬����������ˮ�ĵ��룬�缫��ӦʽΪ��2H2O��4e��=4H����O2������ȷ��C���������缫��Ӧʽ֪��ת��2mol����ʱ������������1molˮ������2molH+���������ң���������������18g���������зų�0.2molN2��5.6g����ͬʱ��2molH+��2g�����������ң������������������3.6g����Ĥ������Һ�������仯���m��-��m������14.4g������D��������ӦʽΪ��2NO3����6H2O��10e����N2����12OH������ȷ��
���㣺������ԭ����Ӧ�ü��缫��Ӧʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ��������������� ���ͣ������
��ѧ�ڻ�������������ʮ����Ҫ�����ã������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
��1�������������У�H2�ܽ�NO3����ԭΪN2��25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12��
��N2�ĽṹʽΪ ��
��������Ӧ�����ӷ���ʽΪ ����ƽ����Ӧ���ʦ�(NO3��)Ϊ mol��L��1min-1��
�ۻ�ԭ�����п������м����NO2����д��3�ִٽ�NO2��ˮ��ķ��� ��
��2���绯ѧ����NO3����ԭ����ͼ��ʾ��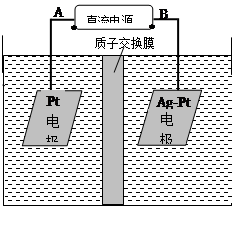
�ٵ�Դ����Ϊ ����A��B����������ӦʽΪ ��
������������ת����2mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ȫ����ͨ�ߵ�ѧУ����ͳһ���������ۺ��������Ի�ѧ������������棩 ���ͣ������
��ѧ�ڻ�������������ʮ����Ҫ�����ã������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
��1�������������У�H2�ܽ�NO3����ԭΪN2��25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12��
��N2�ĽṹʽΪ ��
��������Ӧ�����ӷ���ʽΪ ����ƽ����Ӧ���ʦ�(NO3��)Ϊ mol��L��1min-1��
�ۻ�ԭ�����п������м����NO2����д��3�ִٽ�NO2��ˮ��ķ��� ��
��2���绯ѧ����NO3����ԭ����ͼ��ʾ��

�ٵ�Դ����Ϊ ����A��B����������ӦʽΪ ��
������������ת����2mol���ӣ���Ĥ������Һ�������仯��(��m������m��)Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ�ڻ�������������ʮ����Ҫ�����ã������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
(1) �����������У�H2�ܽ�NO3����ԭΪN2��25��ʱ����Ӧ����10min����Һ��pH��7��Ϊ12��
��N2�ĽṹʽΪ ��
��������Ӧ���ӷ���ʽΪ ����ƽ����Ӧ����v(NO3��)Ϊ ��
|
����NaNO2 ��Һ�У������غ����ʽΪ
___________________________________________��
(2)�绯ѧ����NO3����ԭ������ͼ��ʾ��
| |
������ӦʽΪ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com