

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��Ũ��ˮ | B����ˮ |
| C������K2CO3���� | D������ŨH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
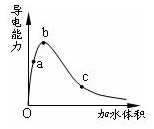
| A��a��b��c������Һ��pH��c��a��b |
| B��a��b��c������Һ��1mol/L����������Һ�кͣ���������������Һ�����c��a��b |
| C��a��b��c������b��n(H+)��� |
| D��a��b��c�������ĵ���̶ȣ�a��b��c |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH3��H2O | B��H2O | C��CH3COOH | D������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
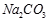 ��
��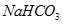 ?��
?��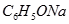 ? ��
? ��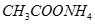 ��
��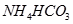 ����֪����
����֪����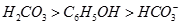 ����
�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һ����ˮ�������c(OH��)=1��10��10mol/L |
| B����Һ��c(OH��)��c��NH3��H2O��=0.1mol/L |
| C����0.1mol/L ������������Ϻ�������Һ�У�c(Cl��)=c(NH4��)��c��NH3��H2O��=0.1mol/L |
| D��ԭ��Һ�м����������Ȼ�茶�����ˮϡ�ͣ���Һ�е�c(H��)������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
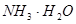 �����
����� ����ֵ����ʵ�鷽����������£�
����ֵ����ʵ�鷽����������£�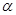 __________��
__________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�0.1mol��L-1��CH3COONa��Һ��pH=8 |
| B�������£�0.1mol��L-1��CH3COOH��Һ��pH="3" |
| C�������м�ˮ����Һ��pH���� |
| D��0.1mol��L-1��CH3COOH������������0.1mol��L-1������ǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com