
(4��)��һƿ�������Һ�����п��ܺ�NH4����K����Na ����Mg2����Ba2���� Al3���� Fe3����SO42����CO32����NO3�� ��Cl����I����ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ��ǿ���ԣ�
��ȡ������Һ����������CCl4���������Ƶ���ˮ��������CCl4����Ϻ�ɫ��
��ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о��������ɣ�
��ȡ���������ʼ��Ե���Һ����Na2CO3��Һ���а�ɫ�������ɣ�
�ɽ��ǵõ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�����Һ�п϶����ڵ��������������� ��������ȷ���Ƿ����������������______________��
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)��һƿ�������Һ�����п��ܺ���NH4����K����Na����Mg2����Ba2����Al3����Fe3����SO42����CO32����Cl����I����ȡ����Һ��������ʵ�飺
(1)��pH��ֽ���飬������Һ��ǿ���ԣ�
(2)ȡ������Һ����������CCl4���������Ƶ���ˮ�������ã���Һ�ֲ㣬�²���Ϻ�ɫ��
(3)��ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о��������ɣ�
(4)ȡ��������������Һ���ȣ���Na2CO3��Һ���а�ɫ�������ɣ�
(5)��(3)�õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵ�жϣ��ڸ���Һ�п϶����ڵ������� ���϶������ڵ������� ������ȷ���Ƿ���ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�������и���8���¿������Ծ����������� ���ͣ������
(16�֣���һƿ�������Һ�����п��ܺ���NH4+��K+��Na+��Mg2+��Al3+��Fe3+��Ba2+��Cl����I����NO3����CO32����SO42���еļ��֡�ȡ����Һ��������ʵ�飺
(1)��pH��ֽ�������Һ��ǿ���ԣ��ų� ���ӵIJ����ڡ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ�������ų�
���Ӳ����ڡ�
(3)��ȡ������Һ����������μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ��������ɣ�����ų� ���Ӳ����ڡ�ȡ���ּ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������˵�� ���Ӵ���
(4)��ȡ��������������Һ�������м���Na2CO3��Һ���а�ɫ�������ɣ������ų� ���Ӳ����ڡ�
(5)��������ʵ����ʵȷ��������Һ�п϶����ڵ������� �������ܿ϶��Ƿ���ڵ������� ��д����(3)�����������ӷ�Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����һ�и�����һ��������⻯ѧ�Ծ� ���ͣ�ʵ����
��16�֣���1����Fe2+��NO-3��Fe3+��NH+4��H+��H2O�������ӣ��ֱ�����ͬһ������ԭ��Ӧ�еķ�Ӧ��������
����д���÷�Ӧ�����ӷ���ʽ����Ҫ����ƽ�� ��
�ڸ÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ ��
�۲���2mol��ԭ����ʱת�Ƶ��ӵ�����Ϊ ��
��2��ʵ��������0.5mol/L��NaCl��Һ500mL��������������
���ձ���100mL��Ͳ��1000 mL����ƿ��500mL����ƿ�ݲ�������������ƽ�������룩����ʱ������ʹ�õ������� ������ţ�����ȱ�ٵ������� ʵ�������õ��������������÷ֱ��� �� ��
mL����ƿ��500mL����ƿ�ݲ�������������ƽ�������룩����ʱ������ʹ�õ������� ������ţ�����ȱ�ٵ������� ʵ�������õ��������������÷ֱ��� �� ��
��3����һƿ�������Һ�����к��д�����NO-3��Fe3+��NH+4��H+��K+��Mg2+��Al3+��SO2-4��Ba2+��CO2-3��Cl-��I-���ֽ�������ʵ�飺
�ٲ�֪��Һ�����ԣ�
��ȡ�����������Ȼ�̼������������ˮ�����Ȼ�̼����Һ���Ϻ�ɫ��
����ȡ���μ�ϡNaOH��Һ��ʹ��Һ��Ϊ���ԣ��˹������������ɣ�
��ȡ��������������Һ����Na2CO3��Һ���ְ�ɫ������
�� ��ʵ����еļ�����Һ���ȣ�������ų���������
��ʵ����еļ�����Һ���ȣ�������ų��������� ��ʹʪ��ĺ�ɫʯ����ֽ������
��ʹʪ��ĺ�ɫʯ����ֽ������
�ɴ˿����ƶϣ�
��Һ�п϶����ڵ������� ��
��Һ�в���ȷ���Ƿ���ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��������е�����ѧ�����ڶ���ģ�⿼�� ���ͣ������
��14��)
��A��B��C��D��E��Ϊ������ˮ�Ĺ��壬������ǵ������У�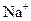 ��
��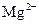 ��
��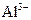 ��
��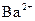 ��
��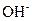 ��
��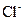 ��
��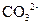 ��
�� ��
��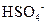 ���ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
���ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
��A��Һ��B��Һ��Ӧ���ɰ�ɫ����������������E��Һ��
��A��Һ��C��Һ��Ӧ���ɰ�ɫ����������������E��Һ��
��A��Һ��D��Һ��Ӧ���ɰ�ɫ�������������������
��B��Һ������D��Һ��Ӧ���ɰ�ɫ�������������D��Һ�����������٣�������ʧ��
�ݴ��ƶ������ǣ�A ��B ��C ��D ��E ��
����һƿ�������Һ�����п��ܺ���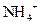 ��
��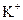 ��
��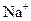 ��
��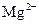 ��
��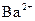 ��
��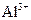 ��
�� ��
��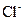 ��
��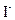 ��
��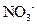 ��
��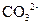 ��
�� �еļ��֡�ȡ����Һ��������ʵ�飺
�еļ��֡�ȡ����Һ��������ʵ�飺
(1)��pH��ֽ���飬������Һ��ǿ���ԡ�
(2)ȡ��������Һ����������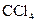 ������������ˮ������
������������ˮ������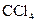 ����Ϻ�ɫ��
����Ϻ�ɫ��
(3)��ȡ������Һ���������м���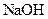 ��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ�����������ȡ���ּ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ�����������ȡ���ּ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
(4)��ȡ��������������Һ�������м���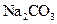 ��Һ���а�ɫ�������ɡ�
��Һ���а�ɫ�������ɡ�
(5)��������ʵ����ʵȷ�����ٸ���Һ�п϶����ڵ������� ���ڻ�����ȷ���Ƿ���ڵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ����8���¿������Ծ��������棩 ���ͣ������
(16�֣���һƿ�������Һ�����п��ܺ���NH4+��K+��Na+��Mg2+��Al3+��Fe3+��Ba2+��Cl����I����NO3����CO32����SO42���еļ��֡�ȡ����Һ��������ʵ�飺
(1)��pH��ֽ�������Һ��ǿ���ԣ��ų� ���ӵIJ����ڡ�
(2)ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ�������ų�
���Ӳ����ڡ�
(3)��ȡ������Һ����������μ���NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ��������ɣ�����ų� ���Ӳ����ڡ�ȡ���ּ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������˵�� ���Ӵ���
(4)��ȡ��������������Һ�������м���Na2CO3��Һ���а�ɫ�������ɣ������ų� ���Ӳ����ڡ�
(5)��������ʵ����ʵȷ��������Һ�п϶����ڵ������� �������ܿ϶��Ƿ���ڵ������� ��д����(3)�����������ӷ�Ӧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com