
 ��ͼ�Dz�����Һ�õ������ǣ�����ʽΪC6H12O6����Է�������Ϊ180��ע��Һ�ı�ǩ�ϵIJ������ݣ�
��ͼ�Dz�����Һ�õ������ǣ�����ʽΪC6H12O6����Է�������Ϊ180��ע��Һ�ı�ǩ�ϵIJ������ݣ� =
= mol���������ǵ����ʵ���Ũ��Ϊ
mol���������ǵ����ʵ���Ũ��Ϊ =0.28mol/L��
=0.28mol/L�� =12.5g�����Ƹ�Ũ����������Һ��Ҫ���������У���Ͳ���ձ�����ͷ�ιܡ���������250mL����ƿ�ȣ�
=12.5g�����Ƹ�Ũ����������Һ��Ҫ���������У���Ͳ���ձ�����ͷ�ιܡ���������250mL����ƿ�ȣ�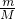 ���������ǵ��������ٸ���c=
���������ǵ��������ٸ���c= ���������ǵ����ʵ���Ũ�ȣ�
���������ǵ����ʵ���Ũ�ȣ� ����һ�����ʵ���Ũ�����ƣ�
����һ�����ʵ���Ũ�����ƣ� 

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��1.2g RSO4�к�0.01mol R2+����RSO4��Ħ��������
��1��1.2g RSO4�к�0.01mol R2+����RSO4��Ħ��������| ���� | �۵�/�� | �е�/�� | �ܶ�/g?cm-3 | �ܽ��� |
| A | -11.5 | 198 | 1.11 | A��B���ܣ��Ҿ�������ˮ |
| B | 17.9 | 290 | 1.26 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�Dz�����Һ�õ������ǣ�����ʽΪC6H12O6����Է�������Ϊ180��ע��Һ�ı�ǩ�ϵIJ������ݣ�
��ͼ�Dz�����Һ�õ������ǣ�����ʽΪC6H12O6����Է�������Ϊ180��ע��Һ�ı�ǩ�ϵIJ������ݣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����н�̨����һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com