
��ҵ�Ͽ������÷����е�CO2Ϊԭ����ȡ�״����䷴Ӧ����ʽΪ��CO2+3H2 CH3OH+H2O����ش��������⣺
CH3OH+H2O����ش��������⣺
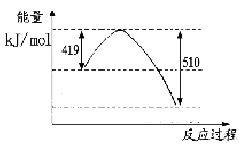
��1����֪���³�ѹ�����з�Ӧ�������仯����ͼ��ʾ��
|
|
|
|

 д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��
д���ɶ�����̼�������Ʊ��״����Ȼ�ѧ����ʽ__ _��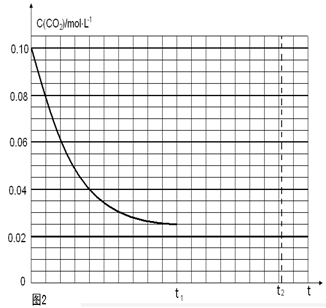
��1��3H2(g) + CO2(g)= CH3OH(l)+ H2O(l)����H=��50KJ/mol��<�� ���£���2��A��D��
��3��CD����4��0.01mol.L-1.min-1����ͼ
���������������1����������ɵ��Ȼ�ѧ����ʽ����CO(g)+H2O(l)=CO2(g)+H2(g) ��H=��41KJ/mol; ��CO(g) +H2(g) =CH3OH(l)��H=��91KJ/mol; �ڣ��٣������ɵ�3H2(g) + CO2(g)= CH3OH(l)+ H2O(l)����H=��50KJ/mol;�ɷ���ʽ��֪���÷�Ӧ��һ����ϵ�Ļ��ҳ̶ȼ�С�ķ�Ӧ�����ԡ�S<0�����ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ�����Է�Ӧ�ڵ�������������ڸ÷�Ӧ�Է����С���2�����ڸ÷�Ӧ������Ӧ�Ƿ��ȷ�Ӧ������������Ӧ����ʽ��ƽ�ⳣ��Kֵ�����ƽ�������ƶ�������Kֻ���¶��йأ�����ѹǿ��Ũ�ȵ��أ�����ֻ���¶Ƚ��Ͳſ�������������������ʱ��V����V�涼��С��V���С�Ķ࣬V��>V�棬ƽ�������ƶ����淴Ӧ�����ȼ�С�����������ӡ����ѡ��ΪA��D����3��A������Ӧ�ﵽƽ�⣬��v��(H2)= 3v��(CO2)������B�����ڶ�������ʱ�ǰ���3:1�����ʵ����Ĺ�ϵ���ĵģ������ڿ�ʼ���������������ֻ�а���ijһȷ���ı�����ϣ��ﵽƽ��ʱ���й�ϵ��C(H2) = C(CO2)����˲�����Ϊ�ж�ƽ��ı�־������C��������������Һ̬���ʣ�����Ӧδ�ﵽƽ�⣬������������ͻᷢ���仯��������ܶ�Ҳ�ᷢ���ı䡣���������������ܶȲ��䣬������Ϊ�ж�ƽ��ı�־����ȷ��D���÷�Ӧ�Ƿ�Ӧǰ������������ȵķ�Ӧ�����δ�ﵽƽ�⣬����������ʵ����ͻᷢ���仯���������������ѹǿ�ͻ�ı䡣���������ѹǿ���������Ϊ�ж�ƽ��ı�־����ȷ����4����Ӧ�������ݻ�Ϊ2.0L��������������ܶ�������2.0g/L�������������������2.0g/L��2.0L=4.0g.�������ӵ�CO2������Ϊ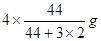 �����Ԧ�n(CO2)=" ��m��M="
�����Ԧ�n(CO2)=" ��m��M="  =0.08mol,���V(CO2)= ��c(CO2)�¦�t="(0.08mol" ��2L)��4min = 0.01mol/(L��min)��
=0.08mol,���V(CO2)= ��c(CO2)�¦�t="(0.08mol" ��2L)��4min = 0.01mol/(L��min)��
��ͼ���֪����ӦCO2(g)+3H2(g) CH3OH(l)+H2O(l)����ʼʱc(CO2)=0.10mol/L��ƽ��ʱc(CO2)=0.025mol/L����c(CO2)=0.075mol/L������ƽ��ʱ�����ʵ�Ũ��Ϊ��c(H2)=0.075mol/L���÷�Ӧ��ƽ�ⳣ��
CH3OH(l)+H2O(l)����ʼʱc(CO2)=0.10mol/L��ƽ��ʱc(CO2)=0.025mol/L����c(CO2)=0.075mol/L������ƽ��ʱ�����ʵ�Ũ��Ϊ��c(H2)=0.075mol/L���÷�Ӧ��ƽ�ⳣ��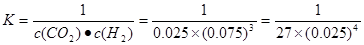 �������������������䣬t1ʱ���������ѹ����1L��c(CO2)=0.05mol/L��c(H2)=0.15mol/L��ƽ�������ƶ���������c(CO2)=xmol/L,������c(H2)=3xmol/L��ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊc(CO2)="(0.05-x)mol/L" ��c(H2)="(0.15-3x)mol/L" ��ƽ�ⳣ�����䡣��
�������������������䣬t1ʱ���������ѹ����1L��c(CO2)=0.05mol/L��c(H2)=0.15mol/L��ƽ�������ƶ���������c(CO2)=xmol/L,������c(H2)=3xmol/L��ƽ��ʱ�����ʵ�Ũ�ȷֱ�Ϊc(CO2)="(0.05-x)mol/L" ��c(H2)="(0.15-3x)mol/L" ��ƽ�ⳣ�����䡣��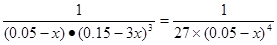 �����x= 0.025mol/L����t2�ﵽ�µ�ƽ��ʱc(CO2)=0.025mol/L.��t1��c(CO2)��ʱ��t�仯��������Ϊ��ͼ��ʾ��
�����x= 0.025mol/L����t2�ﵽ�µ�ƽ��ʱc(CO2)=0.025mol/L.��t1��c(CO2)��ʱ��t�仯��������Ϊ��ͼ��ʾ��
���㣺�����Ȼ�ѧ����ʽ����д����ѧƽ��״̬���жϡ���Ӧ�ķ����ԡ���ѧ��Ӧ���ʵļ��㡢CO2Ũ����ʱ��ͼ��ı�ʾ��


 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ijЩת�����輰������δ�г�����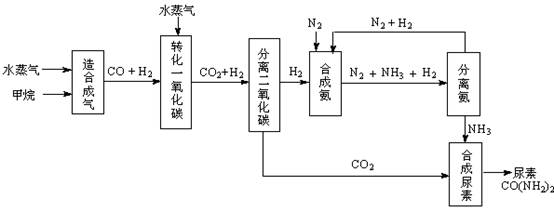
��1������ϳ������������Ȼ�ѧ����ʽ��CH4(g)+H2O(g) 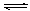 CO(g)+3H2(g)����H��0
CO(g)+3H2(g)����H��0
�ں��º��ݵ������£������CH4�ķ�Ӧ���ʺ�ת���ʣ����д�ʩ���е��� ��
A������ѹǿ B�������¶� C������He�� D������ˮ����Ũ��
��2����ת��һ����̼�������ķ���ʽ��H2O(g) +CO(g) 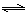 H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
H2(g)+CO2(g)���÷�Ӧƽ�ⳣ�����¶ȵı仯���£�
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ʵ�У�ʲô����Ӱ���˻�ѧ��Ӧ������?
��1������������ʱҪ�����ϸС�Ŀ�����________��
��2���ۻ���KClO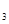 �ų����ݺ�������������MnO
�ų����ݺ�������������MnO �ܿ�������壺________��
�ܿ�������壺________��
��3��ͬŨ�ȡ�ͬ����������з����С��ͬ����Ƭ��þƬ���������п�������________��
��4��ͬ����С��ʯ��ʯ�ֱ���0.1mol/L�������1mol/L�������з�Ӧ���ʲ�ͬ��________________��
��5�������ʳƷ��ù�䣬����Ͳ�������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�������£���Ӧx A��YB zC�ﵽƽ�⣺
zC�ﵽƽ�⣺
��1����A��B��C��Ϊ���壬��÷�Ӧ��ƽ�ⳣ������ʽΪ_______________________��
��2����A��B��C��Ϊ���壬��ѹ��ƽ�����淴Ӧ�����ƶ�����x��y��z��Ĺ�ϵ��___________��
��3����֪C�����壬��x��y��z��������ѹǿʱ����ƽ�ⷢ���ƶ�����һ����________����������桱����Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��16�֣���.������Ϣ��ҵ��̫���ܵ�ع��ת���Ļ������ϡ�п��ԭ���Ȼ�����һ����������Ӧ��ǰ�����Ʊ���ķ��������Ʊ�����ʾ��ͼ���£�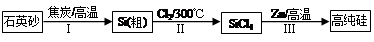
��1����̿�ڹ��̢����� ����
��2�����̢���Cl2�õ�ⱥ��ʳ��ˮ�Ʊ����Ʊ�Cl2�Ļ�ѧ����ʽΪ ��
��3�������������̱����ϸ������ˮ��
��SiCl4��ˮ����ˮ������SiO2��һ���ᣬ��Ӧ����ʽΪ ��
�ڸ���Cl2ʱ�������ڳ�ָ���Ͳ�����ȫ�ĽǶȿ��ǣ��轫Լ90��ij�ʪ��������ȴ��12�棬Ȼ����ͨ��ŨH2SO4�С���ȴ�������� ��
��4��Zn��ԭSiCl4�ķ�Ӧ���£�
��Ӧ�٣�400�桫756�棬SiCl4(g)+2Zn(l) Si(S)+2ZnCl2(l) ��H1��0
Si(S)+2ZnCl2(l) ��H1��0
��Ӧ�ڣ�756�桫907�棬SiCl4(g)+2Zn(l) Si(S)+2ZnCl2(g) ��H2��0
Si(S)+2ZnCl2(g) ��H2��0
��Ӧ�ۣ�907�桫1410�棬SiCl4(g)+2Zn(g) Si(S)+2ZnCl2(g) ��H3��0
Si(S)+2ZnCl2(g) ��H3��0
i. ��Ӧ�ڵ�ƽ�ⳣ������ʽΪ ��
ii. ��������������Ӧ������˵���������� ��
a.�����¶Ȼ����SiCl4��ת���� b.��ԭ�������������������н���
c.����ѹǿ����߷�Ӧ���� d.Na��Mg���Դ���Zn��ԭSiCl4
��5���ù�����̫���ܵ��ʱ��Ϊ�������ڹ����ķ��䣬���û�ѧ��ʴ����������γɴֲڵĶ��㡣��ʴ������ϡHNO3��HF�Ļ��Һ������������γ�SiO2�����ת����H2SiF6���û�ѧ����ʽ��ʾSiO2ת��ΪH2SiF6�Ĺ��� ��
��.��1�����顢������һ����̼��ȼ���ȷֱ�ΪakJ��mol��1��bkJ��mol��1��ckJ��mol��1����ҵ��������ȼ���Ͷ�����̼��Ӧ�Ʊ��ϳ�����CO��H2�������Ȼ�ѧ��Ӧ����ʽΪ ��
��2����֪Ksp(AgCl)��1.8��10��10��Ksp(AgI)��1.5��10��16��Ksp(Ag2CrO4)��2.0��10��12�����������εı�����Һ�У�Ag+Ũ�ȴ�С��˳��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��18�֣�̼������������ѧ��ѧ��Ҫ�ķǽ���Ԫ�أ��ڹ�ũҵ�������й㷺��Ӧ�á�
��1�����ڷ��䡰�칬һ�š��ij�������F�����ȼ����Һ̬ƫ�����£�CH3��NH��NH��CH3������������Һ̬�����������������ڷ�Ӧ�����зų�����������ͬʱ������������Ⱦ�����塣��֪�����£�1 gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��2��298 Kʱ����2L���ܱ������У��������淴Ӧ��2NO2(g) N2O4(g)����H��-a kJ��mol��1 (a>0) ��N2O4�����ʵ���Ũ����ʱ��仯��ͼ����ƽ��ʱ�� N2O4��Ũ��ΪNO2��2�����ش��������⡣
N2O4(g)����H��-a kJ��mol��1 (a>0) ��N2O4�����ʵ���Ũ����ʱ��仯��ͼ����ƽ��ʱ�� N2O4��Ũ��ΪNO2��2�����ش��������⡣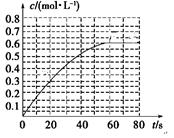
��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ________ L ��mol��1��
��������ʵ���жϸ÷�Ӧ����ƽ��״̬����
a.���������ܶȱ��ֲ���
b.����������ɫ���ٱ仯
c. V��N2O4����=2V��NO2����
������Ӧ��398K���У�ijʱ�̲��n��NO2��="0.6" mol n��N2O4��=1.2mol�����ʱ
V������ V���棩���>����<����=������
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��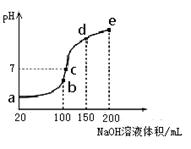
�Է���ͼ��a��b��c��d��e����㣬
��ˮ�ĵ���̶�������__________��
������Һ��c(OH-)����ֵ��ӽ�NH3��H2O�ĵ��볣
��K��ֵ���� ��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳��
��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��16�֣��������ƣ�Na2FeO4�����к�ǿ�������ԣ��㷺Ӧ���ھ�ˮ����ع�ҵ�������Դ�FeO(����CuO��Al2O3��SiO2������)�Ʊ��������Ƶ������������£��ش��������⣺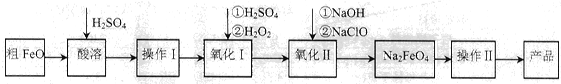
��֪��NaClO���ȶ��������ֽ⡣
��1����FeO���ܹ�����ͨ��ˮ���������£�����Ŀ����__________________________��
��2������IĿ���ǵõ��ߴ���FeSO4��Һ��������I�з�Ӧ�����ӷ���ʽΪ_________��
��3������������Ҫ��Ũ��NaClO��Һ������Cl2��NaOH��Һ��Ӧ�Ʊ�
��Cl2��NaOH��Һ��Ӧ�����ӷ���ʽΪ_________________��
���ڲ�ͬ�¶��½��и÷�Ӧ����Ӧ��ͬһ��ʱ��������NaClOŨ�����£�
| �¶�/�� | 15 | 20 | 25 | 30 | 35 | 40 | 45 |
| NaClOŨ��/mol��L-1 | 4.6 | 5.2 | 5.4 | 5.5 | 4.5 | 3.5 | 2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��6�֣���6 molA�����5 molB�����Ϸ���4L�����ܱ������С���һ�������·�����Ӧ��3A(g)+B(g) 2C(g)+xD(g)������5 min��ﵽ��ѧƽ�⣬��ʱ����2 molC����֪�ڴ�ʱD��ƽ������Ϊ0��15 mol��L-1��min-1��
2C(g)+xD(g)������5 min��ﵽ��ѧƽ�⣬��ʱ����2 molC����֪�ڴ�ʱD��ƽ������Ϊ0��15 mol��L-1��min-1��
��1��ƽ��ʱA�����ʵ���Ũ�ȣ� ��2�� B��ת���ʣ���3�� x��ֵ����Ҫ��д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�״�����Ϊȼ�ϵ�ص�ԭ�ϡ���CH4��H2OΪԭ�ϣ�ͨ�����з�Ӧ���Ʊ��״���
I��CH4(g)+H2O(g) CO(g)+3H2(g) ��H=+206.0kJ?mol��1
CO(g)+3H2(g) ��H=+206.0kJ?mol��1
II��CO(g)+2H2(g) CH3OH(g) ��H=��129.0kJ?mol��1
CH3OH(g) ��H=��129.0kJ?mol��1
��1��CH4(g)��H2O(g)��Ӧ����CH3OH(g)��H2(g)���Ȼ�ѧ����ʽΪ ��
��2����1.0mol CH4��1.0mol H2O(g)ͨ���ݻ�Ϊ100 L�ķ�Ӧ�ң���һ�������·�����ӦI�������һ����ѹǿ��CH4��ת�������¶ȵĹ�ϵ��ͼ��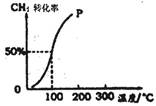
�ټ���100��ʱ�ﵽƽ�����蹹ʱ��Ϊ5min������H2��ʾ�÷�Ӧ��ƽ����Ӧ����Ϊ ��
��1000Cʱ��ӦI��ƽ�ⳣ��Ϊ ��
��3����ѹǿΪ0.1 MPa���¶�Ϊ300�������£���a molCO��2a mol H2�Ļ�������ڴ��������·�����ӦII���ɼ״���ƽ����������ݻ�ѹ����ԭ����1/2�������������䣬��ƽ����ϵ������Ӱ���� ������ĸ��ţ���
| A��ƽ�ⳣ��K���� | B������Ӧ���ʼӿ죬�淴Ӧ���ʼ��� |
| C��CH3OH�����ʵ������� | D������ƽ��c(H2)/c(CH3OH)��С |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com