
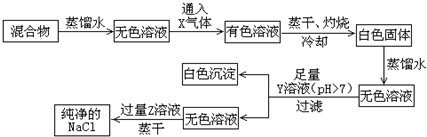
 ��ͼ1��ʢ������Լ�ƿ�ϵı�ǩ�IJ������ݣ���ʵ����Ҫ0.5mol•L-1H2SO4��Һ480mL��������������������Һ�������ʵ�������е�������ҩƷ����ش��������⣺
��ͼ1��ʢ������Լ�ƿ�ϵı�ǩ�IJ������ݣ���ʵ����Ҫ0.5mol•L-1H2SO4��Һ480mL��������������������Һ�������ʵ�������е�������ҩƷ����ش��������⣺���� ��1����������ƿ����ȷʹ�÷������н��
��2������������Һ���ѡ����ʵ�����ƿ����������һ�����ʵ���Ũ�ȵ���Һ����ѡ��������
��3������c=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ���Һϡ�������������ʵ����ʵ������䣬�ݴ˼�����ҪŨ�������������ˮ���ܶȱ�������ܶ�С����������ʱ���������������ˮ������������
��4������ʵ�������c=$\frac{n}{c}$��Ӱ������жϸ����������ƽ����Ӱ�죻
��5�������¶ȼơ�����ƿ���ζ��ܵĹ��켰��ȷʹ�÷������н��
��� �⣺��1������ƿ��©�ķ����Ǽ�����ˮ������ƿ�����ò�©ˮ��Ȼ�����ţ�Ӧע��ƿ��Ҫ��ת180�ȣ��ٵ��ÿ��Ƿ�©ˮ��
�ʴ�Ϊ����ƿ������������ˮ������ƿ������ת��©ˮ��Ȼ�����ţ���ƿ����ת180�ȣ��ٵ�ת��©ˮ����˵��������ƿ��©ˮ��
��2������0.5mol•L-1 H2SO4��Һ480mL��Ӧѡ��500mL����ƿ������һ�����ʵ���Ũ�ȵ���Һ���裺���㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȣ���Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������500mL����ƿ��
����Ͳ���ձ����Ҫ��������������ͷ�ιܡ���������500mL����ƿ��
�ʴ�Ϊ����ͷ�ιܡ���������500mL����ƿ��
��3��Ũ��������ʵ���Ũ��Ϊ��$\frac{1000��1.84��98%}{98}$=18.4mol/L������ҪŨ�������ΪV����������Һϡ�������������ʵ����ʵ������䣺V��18.4mol/L=0.5mol/L��0.5L�����V=0.0136L����13.6mL��ˮ���ܶȱ�������ܶ�С����������ʱ���������������ˮ�����������Ը�������������ˮ���������Һ��������������49%��
�ʴ�Ϊ��13.6������
��4��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�������ȡ����������ʵ���ƫ����ҺŨ��ƫ��A��ȷ��
B��������ƿ��ת��ʱ��û����ȴ���������Ƶ���Һ���ƫС����ҺŨ��ƫ��B��ȷ��
C������ʱ���ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫС����C����
D�����ݺ���ҡ�Ⱥ�������ʱ������Һ����ڿ̶��ߣ��������������������ʵ����ʵ�������Һ�����Ӱ�죬��ҺŨ�Ȳ��䣬��D����
�ʴ�Ϊ��AB��
��5��A����Ͳ��û��0�̶�ֵ����A����
B����Ͳ��ȷֵΪ��0.1mL��ͼ����Һ�����Ϊ2.5mL����B��ȷ��
C���ζ��ܾ�ȷ��Ϊ0.01mL����C����
D��ֻ���¶ȼƵ�0�̶��»������ݣ���ͼʾ�¶�Ϊ2.5�棬��D��ȷ��
�ʴ�Ϊ��BD��
���� ���⿼������Һ���Ƶķ�������Ŀ�Ѷ��еȣ���ȷ����һ�����ʵ���Ũ�ȵ���Һ����Ϊ���ؼ���ע��������������ƿ����Ͳ���ζ��ܵ������Ĺ��켰��ȷʹ�÷���������������ѧ���Ļ�ѧʵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �˵�Ƥ����ǿ�����ߵ������²���ʧȥ�������� | |
| B�� | �������Ƶ�������ͭ����˾���ƺ�ݳ� | |
| C�� | ����ľ�ĵ���Ҫ�ɷֶ�����ά�� | |
| D�� | ���϶�ҧ�˵�Ƥ��ʱ�����������ע�����壬��ʱ���ڻ���ͿĨʳ���ⲻ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Һ©�������Ҵ���ˮ�Ļ��Һ�� | |
| B�� | ������ϴ������������Ӧ���Թ� | |
| C�� | ����ʯ��ʱ���¶ȼƵ�ĩ�˱������Һ���� | |
| D�� | ��������Cu��OH��2��Һʱ����2mL10% CuSO4��Һ�е��뼸��2%NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij�¶��£�0.1mol•L-1�Ĵ�����ҺPH=3��������볣��Ka��1.0��10-4 | |
| B�� | Ka��Kb��Kh�����¶����߶����� | |
| C�� | ����HA�ĵ���ƽ�ⳣ��ΪKa����A-��ˮ��ƽ�ⳣ��ΪKh=Ka•Kw | |
| D�� | ��ΪKsp��AgCl����Ksp��AgOH��������AgCl������ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 30gNO���е�ԭ����ĿΪNA | |
| B�� | ���³�ѹ�£�22.4LH2���еķ�����ĿΪNA | |
| C�� | 5.6g��������������ȫ��Ӧʧȥ�ĵ�����ĿΪ0.2NA | |
| D�� | 1L1mol•L-1Na2SO4��Һ�к��е���������ĿΪ2NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com