
ŅŃÖŖµ„ÖŹĮņŌŚĶس£Ģõ¼žĻĀŅŌS8(Š±·½Įņ)µÄŠĪŹ½“ęŌŚ£¬¶ųŌŚÕōĘųדĢ¬Ź±£¬ŗ¬ÓŠS2”¢S4”¢S6¼°S8µČ¶ąÖÖĶ¬ĖŲŅģŠĪĢ壬ĘäÖŠS4”¢S6ŗĶS8¾ßÓŠĻąĖĘµÄ½į¹¹ĢŲµć£¬Ęä½į¹¹ČēĻĀĶ¼ĖłŹ¾£ŗ
ŌŚŅ»¶ØĢõ¼žĻĀ£¬S8(s)ŗĶO2(g)·¢Éś·“Ó¦ŅĄ“Ī×Ŗ»ÆĪŖSO2(g)ŗĶSO3(g)”£·“Ó¦¹ż³ĢŗĶÄÜĮæ¹ŲĻµæÉÓĆĻĀĶ¼¼ņµ„±ķŹ¾(Ķ¼ÖŠµÄ¦¤H±ķŹ¾Éś³É1 mol²śĪļµÄŹż¾Ż)”£
£Ø1£©Š“³ö±ķŹ¾S8Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½___________________________________”£
£Ø2£©Š“³öSO3·Ö½āÉś³ÉSO2ŗĶO2µÄČČ»Æѧ·½³ĢŹ½_______________________________________________________________”£
£Ø3£©»ÆѧÉĻ¹ę¶Ø£¬²šæŖ»ņŠĪ³É1 mol»Æѧ¼üĪüŹÕ»ņ·Å³öµÄÄÜĮæ³ĘĪŖøĆ»Æѧ¼üµÄ¼üÄÜ£¬µ„Ī»kJ”¤mol”£ČōŅŃÖŖĮņŃõ¼üµÄ¼üÄÜĪŖd kJ”¤mol£1£¬ŃõŃõ¼üµÄ¼üÄÜĪŖe kJ”¤mol£1£¬ŌņS8·Ö×ÓÖŠĮņĮņ¼üµÄ¼üÄÜĪŖ____________________________________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĄūÓƵŖĘų”¢ĒāĘųŌŚŅ»¶ØĢõ¼žĻĀÉś³É°±ĘųÕāŅ»æÉÄę·“Ó¦Ą“ŗĻ³É°±£¬ŹĒŅ»øöÖŲŅŖµÄ»Æ¹¤·“Ó¦”£³£ÓĆĄ“Éś²śŅŗ°±ŗĶ°±Ė®”£
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø1£©ČēĶ¼±ķŹ¾ŗĻ³É°±Ź±Éś³É1molÉś³ÉĪļŹ±µÄÄÜĮæ±ä»Æ£¬EµÄµ„Ī»ĪŖkJ”£ĒėŠ“³öŗĻ³É°±µÄČČ»Æѧ·½³ĢŹ½____________________”£
£ØČČĮæÓĆE1”¢E2»ņE3±ķŹ¾£©”£øĆĶ¼ÖŠµÄŹµĻßÓėŠéĻß²æ·ÖŹĒŹ²Ć“·“Ó¦Ģõ¼ž·¢ÉśĮĖ±ä»Æ£æ
£Ø2£©ŌŚŅ»¶ØĪĀ¶ČĻĀ£¬Čō½«4a mol H2ŗĶ2amol N2·ÅČėVLµÄĆܱÕČŻĘ÷ÖŠ£¬5·ÖÖÓŗó²āµĆN2µÄ×Ŗ»ÆĀŹĪŖ50%£¬ŌņøƶĪŹ±¼äÓĆH2±ķŹ¾µÄ·“Ó¦ĖŁĀŹĪŖ__________Ħ¶ū/(Éż?Ćė)”£Čō“ĖŹ±ŌŁĻņøĆČŻĘ÷ÖŠĶ¶Čėa mol H2”¢amol N2ŗĶ2amol NH3£¬ÅŠ¶ĻĘ½ŗāŅĘ¶ÆµÄ·½ĻņŹĒ_____£ØĢī”°ÕżĻņŅĘ¶Æ”±”°ÄęĻņŅĘ¶Æ”±»ņ”°²»ŅĘ¶Æ”±£©
£Ø3£©Ņŗ°±ŗĶĖ®ĄąĖĘ£¬Ņ²ÄܵēĄė£ŗ2NH3 NH4++ NH2££¬Ä³ĪĀ¶ČŹ±£¬ĘäĄė×Ó»żK=2”Įl0-30”£øĆĪĀ¶ČĻĀ£ŗ¢Ł½«ÉŁĮæNH4Cl¹ĢĢå¼ÓČėŅŗ°±ÖŠ£¬K____________2”Į10-30£ØĢī”°£¼”±”¢”°£¾”±»ņ”°=”±£©£»¢Ś½«ÉŁĮ潚ŹōÄĘĶ¶ČėŅŗ°±ÖŠ£¬ĶźČ«·“Ó¦ŗóĖłµĆČÜŅŗÖŠø÷Ī¢Į£µÄÅØ¶Č“óŠ”¹ŲĻµĪŖ£ŗ_______
NH4++ NH2££¬Ä³ĪĀ¶ČŹ±£¬ĘäĄė×Ó»żK=2”Įl0-30”£øĆĪĀ¶ČĻĀ£ŗ¢Ł½«ÉŁĮæNH4Cl¹ĢĢå¼ÓČėŅŗ°±ÖŠ£¬K____________2”Į10-30£ØĢī”°£¼”±”¢”°£¾”±»ņ”°=”±£©£»¢Ś½«ÉŁĮ潚ŹōÄĘĶ¶ČėŅŗ°±ÖŠ£¬ĶźČ«·“Ó¦ŗóĖłµĆČÜŅŗÖŠø÷Ī¢Į£µÄÅØ¶Č“óŠ”¹ŲĻµĪŖ£ŗ_______
£Ø4£©¹¤³§Éś²śµÄ°±Ė®×÷·ŹĮĻŹ±ŠčŅŖĻ”ŹĶ”£ÓĆĖ®Ļ”ŹĶ0£®1mol/LĻ”°±Ė®Ź±£¬ČÜŅŗÖŠĖę×ÅĖ®ĮæµÄŌö¼Ó¶ų¼õÉŁµÄŹĒ
| A£®c(NH4+)/c(NH3?H2O) | B£®c(NH3?H2O)/c(OH-) |
| C£®c(H+)/c(NH4+) | D£®c(OH-)/c(H+) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚŅ»øöŠ”ÉÕ±Ąļ¼ÓČėŌ¼20 gŅŃŃŠÄ„³É·ŪÄ©µÄĒāŃõ»Æ±µ¾§Ģå[Ba(OH)2”¤8H2O]£¬½«Š”ÉÕ±·ÅŌŚŹĀĻČŅѵĪÓŠ3”«4µĪĖ®µÄ²£Į§Ę¬ÉĻ£¬Č»ŗóĻņÉÕ±ÄŚ¼ÓČėŌ¼10 gĀČ»Æļ§¾§Ģ壬²¢Į¢¼“ÓĆ²£Į§°ōŃøĖŁ½Į°č”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ _____________________________”£
(2)ŹµŃéÖŠŅŖĮ¢¼“ÓĆ²£Į§°ōŃøĖŁ½Į°čµÄŌŅņŹĒ_____________________________”£
(3)Čē¹ūŹµŃéÖŠĆ»ÓŠæ“µ½”°½į±ł”±ĻÖĻó£¬æÉÄܵÄŌŅņŹĒ(“š³öČżøö»ņČżøöŅŌÉĻŌŅņ)__________________________ _____________”£
(4)Čē¹ūĆ»ÓŠæ“µ½”°½į±ł”±ĻÖĻó£¬ĪŅĆĒ»¹æÉŅŌ²ÉČ”ÄÄŠ©·½Ź½Ą“ĖµĆ÷øĆ·“Ó¦ĪüČČ£æ
_______________________________________(“š³öĮ½ÖÖ·½°ø)”£
(5)”°½į±ł”±ĻÖĻóĖµĆ÷øĆ·“Ó¦ŹĒŅ»øö________(Ģī”°·Å³ö”±»ņ”°ĪüŹÕ”±)ÄÜĮæµÄ·“Ó¦”£¼“¶ĻæŖ¾É»Æѧ¼ü________(Ģī”°ĪüŹÕ”±»ņ”°·Å³ö”±)µÄÄÜĮæ________(Ģī”°>”±»ņ”°<”±)ŠĪ³ÉŠĀ»Æѧ¼ü________(Ģī”°ĪüŹÕ”±»ņ”°·Å³ö”±)µÄÄÜĮ攣
(6)øĆ·“Ó¦ŌŚ³£ĪĀĻĀ¾ĶæɽųŠŠ£¬ĖµĆ÷_________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ē°¶ĪŹ±¼äĻƾķĪŅ¹ś“ó²æµÄĪķö²ĢģĘųøųČĖĆĒµÄÉś²śÉś»ī“ųĄ“ĮĖ¼«“óµÄÓ°Ļģ£¬¾ŻĶ³¼ĘĪŅ¹ś²æ·Ö³ĒŹŠĪķö²ĢģÕ¼Č«ÄźŅ»°ė£¬ŅżĘšĪķö²µÄPM2.5Ī¢ĻøĮ£×Ó°üŗ¬(NH4)2SO4”¢NH4NO3”¢½šŹōŃõ»ÆĪļ”¢ÓŠ»śæÅĮ£Īļ¼°Ńļ³¾µČ”£
£Ø1£©ÓŠ»śæÅĮ£ĪļµÄ²śÉśÖ÷ŅŖŹĒÓÉÓŚ²»ĶźČ«Č¼ÉÕµ¼ÖĀµÄĻą¹ŲČČ»Æѧ·½³ĢŹ½ČēĻĀ£ŗ
¢ŁC(s)£«O2(g)=CO2(g)””¦¤H1£½£94kJ”¤mol£1£»
¢ŚC8H16(l)+12O2(g)=8CO2(g)+8H2O(l) ¦¤H2£½£1124kJ”¤mol£1
¢ŪC8H16(l)+4O2=8C£Øg£©+8H2O£Øl£©¦¤H3£½ kJ”¤mol£1
£Ø2£©ÄÉĆ׶žŃõ»ÆīŃæɹā½ā»Ó·¢ŠŌÓŠ»śĪŪČ¾Īļ£ØVOCs£©£¬ČōĪŽĖ®ÕōĘų“ęŌŚ£¬ČżĀČŅŅĻ©½µ½ā·“Ó¦ĪŖ£ŗC2HCl3+2O2”ś2CO2+HCl+Cl2£¬ČōÓŠ×ć¹»ĮæµÄ½µ½āŗóµÄĪ²Ęų£¬ŹµŃéŹŅ¼ģŃé²śĪļÖŠÓŠĀČĘųµÄ¼ņµ„·½·ØŹĒ£ŗ £»ĶعżÖŹĘ×ŅĒ·¢ĻÖ»¹ÓŠ¶ąÖÖø±·“Īļ£¬ĘäÖŠÖ®Ņ»ĪŖ£ŗ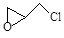 £¬ŌņøĆÓŠ»śĪļŗĖ“Ź²ÕńĒāĘ×ÓŠ ”” øö·å”£
£¬ŌņøĆÓŠ»śĪļŗĖ“Ź²ÕńĒāĘ×ÓŠ ”” øö·å”£
ŅŃÖŖ£ŗCu(OH)2ŹĒ¶žŌŖČõ¼ī£»ŃĒĮ×Ėį£ØH3PO3£©ŹĒ¶žŌŖČõĖį£¬ÓėNaOHČÜŅŗ·“Ó¦£¬Éś³ÉNa2HPO3”£
£Ø3£©ŌŚĶŃĪČÜŅŗÖŠCu2£«·¢ÉśĖ®½ā·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ____£»£ØŅŃÖŖ£ŗ25”ꏱ£¬Ksp[Cu(OH)2]£½2.0”Į10£20mol3/L3£©
£Ø4£©µē½āNa2HPO3ČÜŅŗæɵƵ½ŃĒĮ×Ėį£¬×°ÖĆČēĶ¼£ØĖµĆ÷£ŗŃōĤֻŌŹŠķŃōĄė×ÓĶعż£¬ŅõĤֻŌŹŠķŅõĄė×ÓĶعż£©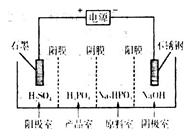
¢ŁŃō¼«µÄµē¼«·“Ó¦Ź½ĪŖ____________________”£
¢Ś²śĘ·ŹŅÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŃŠ¾æCO2µÄĄūÓƶŌ“Ł½ųµĶĢ¼Éē»įµÄ¹¹½Ø¾ßÓŠÖŲŅŖµÄŅāŅ唣
(1)½«CO2Óė½¹Ģæ×÷ÓĆÉś³ÉCO£¬COæÉÓĆÓŚĮ¶ĢśµČ”£
ŅŃÖŖ£ŗFe2O3(s)£«3C(s)=2Fe(s)£«3CO(g)£»¦¤H1£½£«489.0 kJ”¤mol£1
C(s)£«CO2(g)=2CO(g)£»¦¤H2£½£«172.5 kJ”¤mol£1”£
ŌņCO»¹ŌFe2O3µÄČČ»Æѧ·½³ĢŹ½ĪŖ________________________________
(2)ijŹµŃ齫CO2ŗĶH2³äČėŅ»¶ØĢå»żµÄĆܱÕČŻĘ÷ÖŠ£¬ŌŚĮ½ÖÖ²»Ķ¬Ģõ¼žĻĀ·¢Éś·“Ó¦£ŗCO2(g)£«3H2(g) CH3OH(g)£«H2O(g)””¦¤H£½£49.0 kJ”¤mol£1£¬²āµĆCH3OHµÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
CH3OH(g)£«H2O(g)””¦¤H£½£49.0 kJ”¤mol£1£¬²āµĆCH3OHµÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ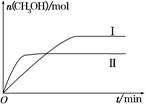
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£ŹżµÄ±ķ“ļŹ½ĪŖK£½________”£
¢ŚĒśĻߢń”¢¢ņ¶ŌÓ¦µÄĘ½ŗā³£Źż“󊔹ŲĻµĪŖK¢ń________K¢ņ(Ģī”°“óÓŚ”±”¢”°µČÓŚ”±»ņ”°Š”ÓŚ”±)”£
¢ŪŌŚĻĀĶ¼a”¢b”¢cČżµćÖŠ£¬H2µÄ×Ŗ»ÆĀŹÓÉøßµ½µĶµÄĖ³ŠņŹĒ________(Ģī×ÖÄø)”£
(3)ŌŚĘäĖūĢõ¼ž²»±äµÄĒéæöĻĀ£¬½«ČŻĘ÷Ģå»żŃ¹Ėõµ½ŌĄ“µÄ1/2£¬ÓėŌĘ½ŗāĻą±Č£¬ĻĀĮŠÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ________(ĢīŠņŗÅ)”£
a£®ĒāĘųµÄÅØ¶Č¼õŠ”
b£®Õż·“Ó¦ĖŁĀŹ¼Óæģ£¬Äę·“Ó¦ĖŁĀŹŅ²¼Óæģ
c£®¼×“¼µÄĪļÖŹµÄĮæŌö¼Ó
d£®ÖŲŠĀĘ½ŗāŹ±n(H2)/n(CH3OH)Ōö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¼×Ķé×÷ĪŖŅ»ÖÖŠĀÄÜŌ“ŌŚ»ÆѧĮģÓņÓ¦ÓĆ¹ć·ŗ£¬Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©øßĀÆŅ±Ģś¹ż³ĢÖŠ£¬¼×ĶéŌŚ“߻Ʒ“Ó¦ŹŅÖŠ²śÉśĖ®ĆŗĘų£ØCOŗĶH2£©»¹ŌŃõ»ÆĢś£¬ÓŠ¹Ų·“Ó¦ĪŖ£ŗCH4£Øg£©£«CO2£Øg£©=2CO£Øg£©£«2H2£Øg£©””¦¤H£½260 kJ”¤mol£1
ŅŃÖŖ£ŗ2CO£Øg£©£«O2£Øg£©=2CO2£Øg£©¦¤H£½£566 kJ”¤mol£1”£
ŌņCH4ÓėO2·“Ӧɜ³ÉCOŗĶH2µÄČČ»Æѧ·½³ĢŹ½ĪŖ____________________________________”£
£Ø2£©ČēĻĀĶ¼ĖłŹ¾£¬×°ÖĆ¢ńĪŖ¼×ĶéČ¼ĮĻµē³Ų£Øµē½āÖŹČÜŅŗĪŖKOHČÜŅŗ£©£¬Ķعż×°ÖĆ¢ņŹµĻÖĢś°ōÉĻ¶ĘĶ”£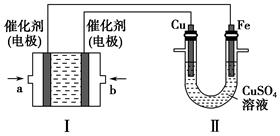
¢Ła“¦Ó¦ĶØČė________£ØĢī”°CH4”±»ņ”°O2”±£©£¬b“¦µē¼«ÉĻ·¢ÉśµÄµē¼«·“Ó¦Ź½ŹĒ_________________________________________________________________”£
¢Śµē¶Ę½įŹųŗó£¬×°ÖĆ¢ńÖŠČÜŅŗµÄpH________£ØĢīŠ“”°±ä“ó”±”°±äŠ””±»ņ”°²»±ä”±£¬ĻĀĶ¬£©£¬×°ÖĆ¢ņÖŠCu2£«µÄĪļÖŹµÄĮæÅضČ________”£
¢Ūµē¶Ę½įŹųŗó£¬×°ÖĆ¢ńČÜŅŗÖŠµÄŅõĄė×Ó³żĮĖOH£ŅŌĶā»¹ŗ¬ÓŠ________£ØŗöĀŌĖ®½ā£©”£
¢ÜŌŚ“Ė¹ż³ĢÖŠČōĶźČ«·“Ó¦£¬×°ÖĆ¢ņÖŠŅõ¼«ÖŹĮæ±ä»Æ12.8 g£¬Ōņ×°ÖĆ¢ńÖŠĄķĀŪÉĻĻūŗļ×Ķé________L£Ø±ź×¼×“æöĻĀ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
CH4”¢H2”¢C¶¼ŹĒÓÅÖŹµÄÄÜŌ“ĪļÖŹ£¬ĖüĆĒČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
¢ŁCH4(g)£«2O2(g)=CO2(g)£«2H2O(l)””¦¤H£½£890.3 kJ”¤mol£1£¬
¢Ś2H2(g)£«O2(g)=2H2O(l)””¦¤H£½£571.6 kJ”¤mol£1£¬
¢ŪC(s)£«O2(g)=CO2(g)””¦¤H£½£393.5 kJ”¤mol£1”£
(1)ŌŚÉīŗ£ÖŠ“ęŌŚŅ»ÖÖ¼×ĶéĻø¾ś£¬ĖüĆĒŅĄææĆøŹ¹¼×ĶéÓėO2×÷ÓĆ²śÉśµÄÄÜĮæ“ę»ī£¬¼×ĶéĻø¾śŹ¹1 mol¼×ĶéÉś³ÉCO2ĘųĢåÓėŅŗĢ¬Ė®£¬·Å³öµÄÄÜĮæ________(Ģī”°£¾”±”°£¼”±»ņ”°£½”±)890.3 kJ”£
(2)¼×ĶéÓėCO2æÉÓĆÓŚŗĻ³ÉŗĻ³ÉĘų(Ö÷ŅŖ³É·ÖŹĒŅ»Ńõ»ÆĢ¼ŗĶĒāĘų)£ŗCH4£«CO2=2CO£«2H2£¬1 g CH4ĶźČ«·“Ó¦æÉŹĶ·Å15.46 kJµÄČČĮ棬Ōņ£ŗ
¢ŁĻĀĶ¼ÄܱķŹ¾øĆ·“Ó¦¹ż³ĢÖŠÄÜĮæ±ä»ÆµÄŹĒ________(Ģī×ÖÄø)”£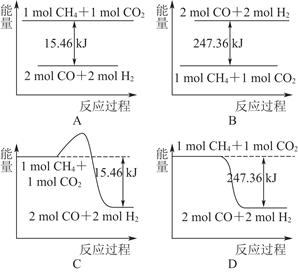
¢ŚČō½«ĪļÖŹµÄĮæ¾łĪŖ1 molµÄCH4ÓėCO2³äČėijŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ĢåĻµ·Å³öµÄČČĮæĖę×ÅŹ±¼äµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņCH4µÄ×Ŗ»ÆĀŹĪŖ________”£
(3)C(s)ÓėH2(g)²»·“Ó¦£¬ĖłŅŌC(s)£«2H2(g)=CH4(g)µÄ·“Ó¦ČČĪŽ·ØÖ±½Ó²āĮ棬µ«ĶعżÉĻŹö·“Ó¦æÉĒó³ö£¬C(s)£«2H2(g)=CH4(g)µÄ·“Ó¦ČȦ¤H£½________”£
(4)ÄæĒ°¶ŌÓŚÉĻŹöČżÖÖĪļÖŹµÄŃŠ¾æŹĒČ¼ĮĻŃŠ¾æµÄÖŲµć£¬ĻĀĮŠ¹ŲÓŚÉĻŹöČżÖÖĪļÖŹµÄŃŠ¾æ·½ĻņÖŠæÉŠŠµÄŹĒ________(Ģī×ÖÄø)”£
| A£®Ń°ÕŅÓÅÖŹ“߻ƼĮ£¬Ź¹CO2ÓėH2O·“Ӧɜ³ÉCH4ÓėO2£¬²¢·Å³öČČĮæ |
| B£®Ń°ÕŅÓÅÖŹ“߻ƼĮ£¬ŌŚ³£ĪĀ³£Ń¹ĻĀŹ¹CO2·Ö½āÉś³ÉĢ¼ÓėO2 |
| C£®Ń°ÕŅÓÅÖŹ“߻ƼĮ£¬ĄūÓĆĢ«ŃōÄÜŹ¹“óĘųÖŠµÄCO2Óėŗ£µ×æŖ²ÉµÄCH4ŗĻ³ÉŗĻ³ÉĘų(CO”¢H2) |
| D£®½«¹ĢĢ¬Ģ¼ŗĻ³ÉĪŖC60£¬ŅŌC60×÷ĪŖČ¼ĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µ½ÄæĒ°ĪŖÖ¹£¬ÓÉ»ÆѧÄÜ×Ŗ±äĪŖČČÄÜ»ņµēÄÜČŌČ»ŹĒČĖĄąŹ¹ÓĆ×īÖ÷ŅŖµÄÄÜŌ“”£
£Ø1£©»Æѧ·“Ó¦ÖŠ·Å³öµÄČČÄÜ£ØģŹ±ä£¬¦¤H£©Óė·“Ó¦ĪļŗĶÉś³ÉĪļŌŚ·“Ó¦¹ż³ĢÖŠ¶Ļ¼üŗĶŠĪ³ÉŠĀ¼ü¹ż³ĢÖŠĪüŹÕŗĶ·Å³öÄÜĮæµÄ“óŠ”ÓŠ¹Ų”£
ŅŃÖŖ£ŗH2£Øg£©£«Cl2£Øg£©=2HCl£Øg£© ¦¤H£½£185 kJ/mol£¬¶ĻĮŃ1 mol H”ŖH¼üĪüŹÕµÄÄÜĮæĪŖ436 kJ£¬¶ĻĮŃ1 mol Cl”ŖCl¼üĪüŹÕµÄÄÜĮæĪŖ247 kJ£¬ŌņŠĪ³É1 mol H”ŖCl¼ü·Å³öµÄÄÜĮæĪŖ ”£
£Ø2£©Č¼ĮĻČ¼ÉÕ½«ĘäĖłŗ¬µÄ»ÆѧÄÜ×Ŗ±äĪŖĪŅĆĒĖłŠčŅŖµÄČČÄÜ”£ŅŃÖŖ£ŗ
¢ŁCH4£Øg£©£«2O2£Øg£©=CO2£Øg£©£«2H2O£Øl£© ¦¤H£½£890£®3 kJ”¤mol-1
¢ŚC£Øs,ŹÆÄ«£©£«O2£Øg£©=CO2£Øg£© ¦¤H£½£393£®5 kJ”¤mol£1
¢Ū2H2£Øg£©£«O2£Øg£©=2H2O£Øl£© ¦¤H£½£571£®6 kJ”¤mol-1
±ź×¼×“æöĻĀ22£®4 LĒāĘųŗĶ¼×ĶéµÄ»ģŗĻĘųĢåŌŚ×ćĮæµÄŃõĘųÖŠ³ä·ÖČ¼ÉÕ·“Ó¦·Å³ö588£®05 kJµÄČČĮ棬Ō»ģŗĻĘųĢåÖŠĒāĘųµÄÖŹĮæŹĒ ”£øł¾ŻŅŌÉĻČżøöČČ»Æѧ·½³ĢŹ½£¬¼ĘĖćC£Øs,ŹÆÄ«£©£«2H2£Øg£©=CH4£Øg£©µÄ·“Ó¦ČȦ¤HĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com