
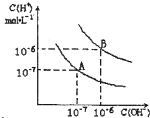
��֪ˮ��25 ���95 ��ʱ�������ƽ����������ͼ��ʾ��
����25 ��ʱˮ�ĵ���ƽ������ӦΪ ___ ���A����B��������˵������ ________________ ��
��95 ��ʱPH=2��NaOH��Һ����ˮ�����c(H��) ______��
��25 ��ʱ����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7����NaOH��Һ��H2SO4��Һ�������Ϊ _________ ��
��25 ��ʱ����100���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ�� _____ ��
������B��Ӧ�¶��£�pH=2��ijHA��Һ��pH=10��NaOH��Һ�������Ϻ����Һ��pH=5�������ԭ�� ��
��95 ��ʱ��ij��ˮ���Ȼ�淋Ļ����Һ��PH=7����c��NH4+��______c��Cl�������>������<������=����
��14�֣��Ţ�A��2�֣���ˮ�ĵ��������ȹ��̣��¶ȵ�ʱ������̶�С��c��H+����c��OH-��С��2�֣���10-10��2�֣���10��1 ��2�֣���a+b=16��pH1+pH2=16��2�֣�
������B��Ӧ95 �棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5����2�֣���>��2�֣�
���������������1�����������ȣ��������¶ȣ�����̶����ӣ�ˮ��������Ũ�����ӣ�����25 ��ʱˮ�ĵ���ƽ������ӦΪA��
��2��95 ��ʱˮ�����ӻ�������10��12������pH=2��NaOH��Һ����ˮ�����c(H��)��10-10mol/L��
��3�������û����Һ��pH=7���� �����
����� ��
��
��4����100���pH1=a��ijǿ����Һ��1���pH2=b��ijǿ����Һ��Ϻ���Һ�����ԣ��� �����Ը�ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��a+b=16��pH1+pH2=16��
�����Ը�ǿ���pH1��ǿ���pH2֮��Ӧ����Ĺ�ϵ��a+b=16��pH1+pH2=16��
��5������B��Ӧ95 �棬��ʱˮ�����ӻ�Ϊ10-12��HAΪ���ᣬHA�к�NaOH�����Һ�л�ʣ��϶��HA���ӣ��ɼ��������H+��ʹ��ҺpH=5��
��6��95 ��ʱ��ij��ˮ���Ȼ�淋Ļ����Һ��PH=7������Һ�Լ��ԡ����Ը��ݵ���غ��֪c��NH4+����c��H+����c��OH������c��Cl������c��NH4+��>c��Cl������
���㣺�������������ˮ�ĵ���ƽ���Ӱ�졢pH�ļ����Լ���Һ������Ũ�ȴ�С�Ƚ�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ������֪ʶ�Ĺ�����ѵ��������������ѧ�������������������ѧ��������û���֪ʶ���ʵ�����������������Ĺؼ�����ȷ�����¶ȵ����ߣ�ˮ�����ӻ������������ڼ���pHʱ��Ҫ������á�


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
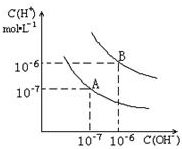
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
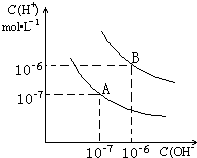 ��֪ˮ��25���99��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���99��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
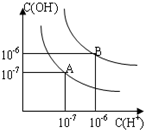 ��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ��
��֪ˮ��25���95��ʱ�������ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����ص�һ��ѧ�߶���ѧ����ĩ������⻯ѧ�Ծ� ���ͣ������
��6�֣���֪ˮ��25���99��ʱ�������ƽ����������ͼ��ʾ��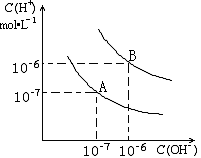
��1����25 ʱˮ�ĵ���ƽ������ӦΪ______(A��B)��
��2��25ʱ������9��NaOH��Һ�룽4��H2SO4��Һ��ϣ������û����Һ�ģ�7����NaOH��Һ��H2SO4��Һ�������Ϊ____________��
��3��99ʱ����100���1����ijǿ����Һ��1���
2��b��ijǿ����Һ��Ϻ���Һ�����ԣ�����ǰ����b֮��Ӧ����Ĺ�ϵ��____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com