
����˵����ȷ����(����)
A����101 kPaʱ��1 mol H2��ȫȼ��������̬ˮ���ų�285.8 kJ������H2��ȼ����Ϊ��285.8 kJ·mol��1
B���ⶨHCl��NaOH��Ӧ���к���ʱ��ÿ��ʵ���Ӧ����3���¶ȣ���������ʼ�¶ȣ�NaOH��ʼ�¶Ⱥͷ�Ӧ����ֹ�¶�
C����101 kPaʱ��1 mol C������O2��Ӧ����1 mol COʱ���ų�110.5 kJ��������C��ȼ����Ϊ110.5 kJ·mol��1
D����ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l)����H����57.31 kJ·mol��1��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼�⻯������һ�ִ�����Ⱦ���������IJ�����̼�⻯�����йص���(����)
A�������ն� B���⻯ѧ����
C������ D����ɽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���е������Һ����Na2CO3����NaHCO3����NaAlO2����CH3COONa����NaOH������֪��CO2��3H2O��2AlO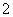 ===2Al(OH)3����CO
===2Al(OH)3����CO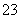 ��
��
(1)��������Һ��pH��ͬʱ�������ʵ���Ũ���ɴ�С��˳����__________________��
(2)���������ʵ���Ũ�Ⱦ�Ϊ0.1 mol·L��1��������Һ��ϡ����ͬ����ʱ����pH�仯������________(����)��
(3)���������ֵ������Һ�У��ֱ����AlCl3��Һ���������������________(����)��
(4)�������٢ڢۢ����ֵ������Һ���ʱ��������Ӧ�����ӷ���ʽΪ_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���̶��ݻ����ܱ������г���2 mol A��1 mol B��������Ӧ��2A(g)��B(g)xC(g)���ﵽƽ���C���������Ϊw%����ά������������¶Ȳ��䣬��0.6 mol A��0.3 mol B��1.4 mol C����������У���ƽ���C���������ҲΪw%����xֵΪ(����)
A��ֻ��Ϊ2
B��ֻ��Ϊ3
C��������2��Ҳ������3
D����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��֪��2NO2(g)N2O4(g)����H����57.20 kJ·mol��1��һ���¶��£����ܱ������з�Ӧ 2NO2(g)N2O4(g)�ﵽƽ�⡣
������������ʱ�����д�ʩ�����NO2��ת���ʵ���________(����ĸ)��
A����СNO2��Ũ�ȡ����� B�������¶�
C������NO2��Ũ�� D�������¶�
(2)17 �桢1.01��105Pa���ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ��c(NO2)��0.030 0 mol·L��1��c(N2O4)��0.012 0 mol·L��1�����㷴Ӧ2NO2(g)N2O4(g)��ƽ�ⳣ��K��
(3)����һ������Cu��������ŨHNO3��Ӧ���Ƶ�1.00 L�Ѵﵽƽ���N2O4��NO2�Ļ������(17 �桢1.01��105Pa)������������������Cu���ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����(����)
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g)����H����49.0 kJ·mol��1
��CH3OH(g)�� O2(g)===CO2(g)��2H2(g)����H����192.9 kJ·mol��1
O2(g)===CO2(g)��2H2(g)����H����192.9 kJ·mol��1
����˵����ȷ����(����)
A��CH3OH�ı�ȼ����Ϊ��192.9 kJ·mol��1
B����Ӧ���е������仯��ͼ��ʾ
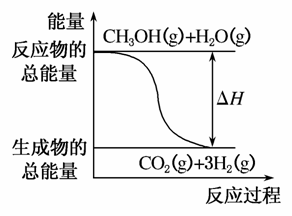
C��CH3OHת���H2�Ĺ���һ��Ҫ��������
D�����ݢ���֪��ӦCH3OH(l)�� O2(g)===CO2(g)��2H2(g)�Ħ�H>��192.9 kJ·mol��1
O2(g)===CO2(g)��2H2(g)�Ħ�H>��192.9 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Դ��������������ͷ�չ����Ҫ���ʻ�����������Դ�ĺ������ú�����Դ�Ŀ����ǵ�������������ٵ��Ͼ����⡣�ش��������⣺
(1)�ҹ���������������úΪ��Ҫȼ�ϵĹ��ң����й���ú��ȼ�ϵ��۵���ȷ����(����)
A��ú����Ҫ�Ļ���ԭ�ϣ���ú��ȼ�ϼ�ȼ�յ�̫��ϧ��Ӧ���ۺ�����
B��ú�Ƿ��ȺܸߵĹ���ȼ�ϣ��ҹ�ú̿��Դ��Լ��У����ɳɱ��ͣ���ú��ȼ�Ϻ���
C��úȼ��ʱ������������������̳����Ի�����Ⱦ����
D��ͨ���ྻú��������ú��������Һ���Լ�����������������ȼú��Ⱦ���������úȼ�յ���������
(2)�ڿ�����ѧ�ҽ�ͭ��������ۻ��Ƴɶ��������������̫�ջ����ʹ�õ�ú��ȼ��������������������������________________________________________________________��
(3)�Ҵ���δ����ȼ������ѡ������Һ��ȼ�ϣ�����������ɫֲ��Ľո���ȡ��1.0 g�Ҵ���ȫȼ������Һ̬ˮ�ų�29.72 kJ��������ʾ�Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�߷��Ӳ���PET������֬��PMMA�ĺϳ�·�����£�

��֪����.RCOOR�䣫R��18OH RCO18OR�士R��OH(R��R�䡢R���������)
RCO18OR�士R��OH(R��R�䡢R���������)

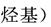
(1)�ٵķ�Ӧ������________��
(2)�ڵĻ�ѧ����ʽΪ________________________________________��
(3)PMMA���������������_________________________________��
__________________��
(4)F�ĺ˴Ź���������ʾֻ��һ��壬�ݵĻ�ѧ����ʽΪ______________��
(5)G�Ľṹ��ʽΪ________��
(6)����˵����ȷ����________(����ĸ���)��
a����Ϊ������Ӧ
b��B��D��Ϊͬϵ��
c��D�ķе��̼ͬԭ������������
d��1 mol 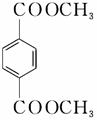 ������NaOH��Һ��Ӧʱ���������4 mol NaOH
������NaOH��Һ��Ӧʱ���������4 mol NaOH
(7)J��ij��ͬ���칹����J������ͬ�����ţ���Ϊ˳ʽ�ṹ����ṹ��ʽ��___________________________________��
(8)д����PET�����Ʊ�PET����������B�Ļ�ѧ����ʽ��_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������������¿��Է���ˮ�ⷴӦ������������A��B����A��B����Է���������ȣ������ʿ�����(����)

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com