

| 0.764376g |
| 0.7759g |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

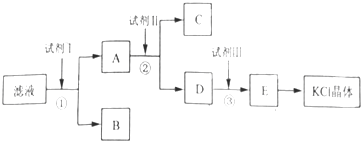
(1)������Ʒ����ʱ����Ӧ�Ļ�ѧ����ʽΪ___________________��
(2)�ܷ���Ba(NO3)2����BaCl2________,������_______________________________��
(3)֤��![]() ������ȫ�ķ�����___________________________________________��
������ȫ�ķ�����___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���¿α���������¿����ģ���ѧ�Ծ���A�����������棩 ���ͣ��ƶ���
[2012��ȫ����]��12�֣��Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ٽ���Һ����ͼ��ʾ������в�����
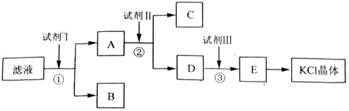
�ش��������⣺
��1����ʼ��Һ��pH_ _7(����ڡ�����С�ڡ����ڡ�)����ԭ����____________��
��2���Լ���Ļ�ѧʽΪ______�����з�����Ӧ�����ӷ���ʽΪ___________ ______��
��3���Լ���Ļ�ѧʽΪ______�����м����Լ����Ŀ����____________ ________��
��4���Լ����������________�����з�����Ӧ�����ӷ���ʽΪ_________ ____��
��5��ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.775 9 g���ܽ������100 mL����ƿ�У�ÿ��ȡ25.00 mL��Һ����0.100 0 mol��L��1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62 mL���ò�Ʒ�Ĵ���Ϊ_________________________________(��ʽ��������)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com