
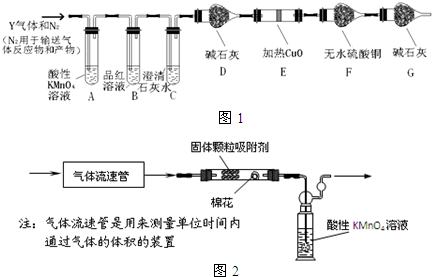
���� ��1��̼�ظַ���Ũ�����У��ڼ���ʱ������Ӧ��2Fe+6H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Fe2��SO4��3+3SO2��+6H2O��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O�����ŷ�Ӧ�Ľ��У�������Һ��ϡ��ᷢ����Ӧ��Fe+H2SO4=FeSO4+H2������Ӧ��õ�����ҺX�к����������������������ᣬ����Y�к���SO2��CO2��ˮ�������ڼ���ʱҪע���ų� SO32-��CO32- �����ӵ�Ӱ�죬�ȼ������ų��������ӣ��ټ��Ȼ���������������ӣ�
��2��̼�ܺ�Ũ���ᷴӦ���ɶ�����������̼��ˮ��
��3����ˮ����ͭ����ˮ��������ɫ������
��4��ȷ������Y�к���CO2��Ӧ��ȫ�ų���������ĸ��ţ����ݶ��������Ư���Խ��
��5��A������ƿ������ˮϴ�Ӻ�δ���T����������Һ�����ڲ�Ӱ�����ʵ����ʵ�������Һ�������
B������ʱ���۲�Һ�温�ӿ̶��ߣ�����Һ�����ƫС��
C��ҡ�Ⱥ�Һ����ڿ̶��ߣ�û���ټ�����ˮ������������
D��������ˮϴ���ձ��Ͳ�����������ϴ��Һת��������ƿ�У�������ȷ��
��6����ʵ�����Ѿ�֪������������������a L/min������KMnO4��Һ�����b L����Ũ��Ϊc mol/L����������ͨ�뵽��ɫǡ����ȥ����ʱ5���ӣ�������������V=5aL������SO2��KMnO4��Ӧ�ķ���ʽ��2KMnO4+5SO2+2H2O=K2SO4+2MnSO4+2H2SO4��֪n��SO2��=$\frac{5}{2}$n��KMnO4��=2.5bcmol���Դ˼���˴�ȡ�����Ŀ����ж�����������
��� �⣺��1���ټ��� SO42- ʱҪ���� SO32- ��Ӱ�죬�Ͳ�Ҫ����ϡ���ᣬ��Ϊϡ�����ܰ�SO32- ����ΪSO42-���Ӷ�����SO42- �ļ��飻Ҫ�ȼ������ϡ���ᣬ�Գ�ȥ SO32-��CO32- �����ӵ�Ӱ�죬ͬʱ��������ϡ����ʱû�г��������ų�Cl-��Ӱ�죮��Ȼ������Һ�в���SO32-���ӣ������ϡ����Ҳ�ɣ�����SO42-����BaCl2��Һ������û�а�ɫ�������������Ϸ�����֪������ij��Һ���Ƿ���SO42-�IJ��������ǣ���������Һ�м��������ữ���ټ���BaCl2��Һ������BaSO4��ɫ������������֤����SO42-��������ҺX�к���SO42-���Լ���HCl��BaCl2��
�ʴ�Ϊ��HCl��BaCl2��
��2��̼�ܺ�Ũ���ᷴӦ���ɶ�����������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
�ʴ�Ϊ��C+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CO2��+2SO2��+2H2O��
��3��װ��F�������Ǽ����������е�ˮ��������ˮ����ͭ����ˮ��������ɫ��
�ʴ�Ϊ�������Ƿ���ˮ����������
��4��ȷ������Y�к���CO2��Ӧ��ȫ�ų���������ĸ��ţ���B��Ʒ����Һ����ɫ��C��ʯ��ˮ����ǣ���˵������CO2��
�ʴ�Ϊ��B��Ʒ�첻��ɫ��C�г���ʯ��ˮ����ǣ�
��5��A������ƿ������ˮϴ�Ӻ�δ���T����������Һ�����ڲ�Ӱ�����ʵ����ʵ�������Һ����������Զ����Ƶ���Һ��Ũ�Ȳ������κ�Ӱ�죬�ʲ�ѡ��
B������ʱ���۲�Һ�温�ӿ̶��ߣ�����Һ�����ƫС���������Ƶ���Һ��Ũ��ƫ��ѡ��
C��ҡ�Ⱥ�Һ����ڿ̶��ߣ�û���ټ�����ˮ�������Һ��Ũ�Ȳ������ʲ�ѡ��
D��������ˮϴ���ձ��Ͳ�����������ϴ��Һת��������ƿ�У�������ȷ���ʲ�ѡ��
�ʴ�Ϊ��B��
��6����ʵ�����Ѿ�֪������������������a L/min������KMnO4��Һ�����b L����Ũ��Ϊc mol/L����������ͨ�뵽��ɫǡ����ȥ����ʱ5���ӣ�������������V=5aL������SO2��KMnO4��Ӧ�ķ���ʽ��2KMnO4+5SO2+2H2O=K2SO4+2MnSO4+2H2SO4��֪n��SO2��=$\frac{5}{2}$n��KMnO4��=2.5bcmol����˴�ȡ�����Ŀ����ж���������Ϊ$\frac{2.5bcmol��64g/mol}{5aL}$=$\frac{32bc}{a}$g/L��
�ʴ�Ϊ��$\frac{32bc}{a}$��
���� ���⿼�����ʵ�����ʵ�鼰�Ʊ�ʵ�飬Ϊ��Ƶ���㣬���ջ�ѧʵ��������������ʲ�������Ũ����ķ�Ӧ��ʵ�鷽������ơ����ӵļ��鷽�������ʳɷֵ�ȷ�������������ɳɷֵĺ����ļ����֪ʶΪ���Ĺؼ������ط�����ʵ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
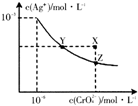 ��֪t��ʱ��AgCl��Ksp=2��10-10��Ag2CrO4���Ⱥ�ɫ���壩��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ���ǣ�������
��֪t��ʱ��AgCl��Ksp=2��10-10��Ag2CrO4���Ⱥ�ɫ���壩��ˮ�еij����ܽ�ƽ��������ͼ��ʾ������˵����ȷ���ǣ�������| A�� | t��ʱ��AgCl��ˮ�е��ܽ�ȱ���ϡ������С | |
| B�� | t��ʱ��AgCl���ܽ�ȴ���Ag2CrO4 | |
| C�� | �ڱ���Ag2CrO4��Һ�м�������K2CrO4����ʹ��Һ��Y������X�� | |
| D�� | ��ͬŨ��NaCl��K2CrO4���Һ�У��μ�0.1mol•L-1AgNO3��Һ�������ɰ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2+2OH-��CO32-+H2O | B�� | Al2O3+2OH-+3H2O��2[Al��OH��4]- | ||
| C�� | 2Al+2OH-+6H2O��2[Al��OH��4]-+3H2�� | D�� | Al3++4OH-��[Al��OH��4]- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� | 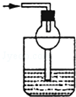 | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢ� | B�� | ֻ�Т� | C�� | �ۢܢ� | D�� | ֻ�Т٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 A��B��C���ֹ������ʵ��ܽ��������ͼ��ʾ��
A��B��C���ֹ������ʵ��ܽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢ� | B�� | �٢� | C�� | �٢ڢۢ� | D�� | �ܢݢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
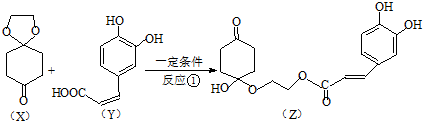
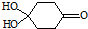 �����Ӽ���ˮ���ã�һ�������£�M����1��-OH����ȥ��Ӧ�õ��ȶ�������N������ʽΪC6H8O2������N�Ľṹ��ʽΪ
�����Ӽ���ˮ���ã�һ�������£�M����1��-OH����ȥ��Ӧ�õ��ȶ�������N������ʽΪC6H8O2������N�Ľṹ��ʽΪ ����֪ϩ��ʽ���ȶ����ᷢ���������ţ����磺
����֪ϩ��ʽ���ȶ����ᷢ���������ţ����磺 ����
����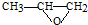 ���������Ʒ�Ӧ�ٵķ�Ӧ����������Ľṹ��ʽΪ
���������Ʒ�Ӧ�ٵķ�Ӧ����������Ľṹ��ʽΪ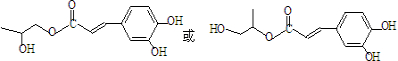 ��дһ�֣���
��дһ�֣����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com