
 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���û�ѧ��Ӧ�Ĺ��������ȵ� |
| B���û�ѧ��Ӧ�Ĺ����Ƿ��ȵ� |
| C����Ӧ����ӵ��������ӣ�����Ӱٷ���������Ч��ײ�������� |
| D�������˶����ʼӿ죬ʹ�÷�Ӧ����ӵ���ײ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� |
B��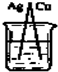 |
C�� |
D��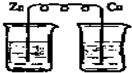 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ϩ��������8���Ҽ���1���м� |
| B����SiO2�����У�1��Siԭ�Ӻ�2��Oԭ���γ�2�����ۼ� |
| C��NF3�ķе��NH3�ķе�͵ö࣬����ΪNH3���Ӽ��������NF3ֻ�з��»��� |
| D��NCl3��BC13�����У�����ԭ�Ӷ�����sp3�ӻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ԣ�B2��C2��A2 |
| B���ں���C-��A-����Һ�м���B2��C-���ȷ�����Ӧ |
| C����ԭ�ԣ�B-��C-��A- |
| D���ں���B2��C2����Һ�м���A-��B2���ȷ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����NaOH��AlCl3 �� MgCl2 ���ֹ�����ɵĻ������������ˮ�У���1.16g��ɫ������������������Һ����μ���1mol?L-1�����ᣬ�������������ͳ�����������ͼ��ʾ��
����NaOH��AlCl3 �� MgCl2 ���ֹ�����ɵĻ������������ˮ�У���1.16g��ɫ������������������Һ����μ���1mol?L-1�����ᣬ�������������ͳ�����������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������þ�еμ����H++OH-�TH2O |
| B�������������Һ�м�������������Һ��pH=7��H++SO42-+Ba2++OH-�TBaSO4��+H2O |
| C����֪����ƽ�ⳣ����H2CO3��HClO��HCO3-�������������Һ��ͨ������������̼�����ӷ���ʽΪ��2ClO-+CO2+H2O�T2HClO+CO32- |
| D����NH4Al��SO4��2��Һ�е���Ba��OH��2��Һ��ǡ��ʹSO42-��ȫ������NH4++Al3++2SO42-+2Ba2++4OH-�TAl��OH��3��+NH3?H2O+2BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com