
| A������ѧʵ���ڼ���봩���䡢��ϥ���·���������Ь�������������죩�������̻����ñ�� |
| B��Ƥ���ϲ���մ��Ũ����Ҫ����������������Һ��ϴ |
| C�����Խ�ֹ��ȼ�ŵľƾ��������Ӿƾ� |
| D����Ϥ����Σ�ջ�ѧƷ��־����Ⱦ�������Ĵ������� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״���£�22.4L��CCl4�к��еķ�����ΪNA |
| B��18gD2O�к��еĵ�����ΪNA |
| C��1L1 mol/L��������Һ�����ķ�����ΪNA |
| D��4 g������������������Ϊ2 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��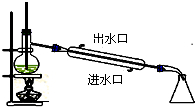 ʯ������ |
B��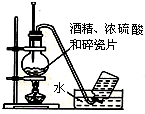 ʵ�������Ҵ���ȡ��ϩ |
C��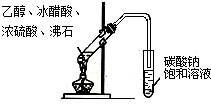 ʵ������ȡ�������� |
D��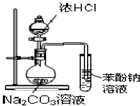 ֤�����ԣ����̼����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ƽ��ʵ���� | Na2C2O4���� ��m g�� | �ζ�����ʼ������mL�� | �ζ��ܵζ��յ������mL�� |
| 1 | 1.34 | 0.00 | 25.02 |
| 2 | 1.34 | 0.02 | 25.00 |
| 3 | 1.34 | 0.18 | 25.18 |
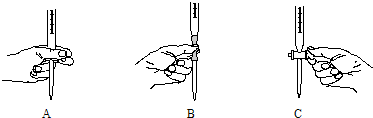
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
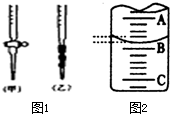 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.52 | 25.42 |
| �ڶ��� | 20.00 | 4.07 | 29.17 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
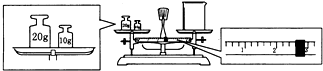 ʵ��������NaOH��������1.0mol?L-1��NaOH��Һ240mL��
ʵ��������NaOH��������1.0mol?L-1��NaOH��Һ240mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
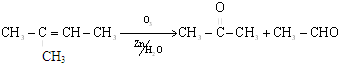 ��ʯ�ͷ����ƷC8H18��ȡ��������ͪ����������ϣ���������ͼ������ע��E��D��һԪȡ���
��ʯ�ͷ����ƷC8H18��ȡ��������ͪ����������ϣ���������ͼ������ע��E��D��һԪȡ���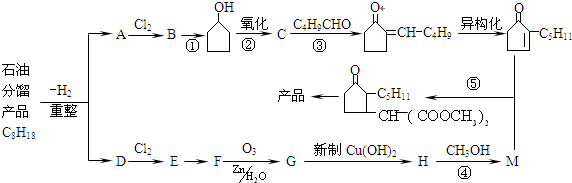
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com