
����Ŀ��ʵ�����Ʊ���ˮ����ͭ[Cu(HCOO)24H2O]����ʵ�鲽�����¡�
(1)��ʽ̼��ͭ���Ʊ���
a.����i�ǽ�һ����������̼�����ƹ���һ��ŵ��в�����ĥ����Ŀ����______��
b.����ii���ڽ����½���������ֶ�λ���������ˮ�У���Ӧ�¶ȿ�����70�桪80�棬�������_____(дʵ������)��˵���¶ȹ��ߡ�
c.��صĻ�ѧ����ʽ______��
(2)��ˮ����ͭ������Ʊ�������ʽ̼��ͭ��������ձ��У�����һ�����ȵ�����ˮ������μ����������ʽ̼��ͭǡ��ȫ���ܽ⣬���ȹ��˳�ȥ�������������ʣ�Ȼ��������ȴ���ˣ�����������ˮ�Ҵ�ϴ�Ӿ���2��3�����ɣ��õ���Ʒ��
a.��صĻ�ѧ����ʽ______��
b.���ȹ����У�������ȵ�ԭ����______��
c.���Ҵ�ϴ�Ӿ����Ŀ��______��
���𰸡���ϸ����Ͼ��� �к�ɫ�������� ![]()
![]() ��ֹ����ͭ�������� ϴȥ��������ˮ���������ʣ����ټ���ͭ����ʧ
��ֹ����ͭ�������� ϴȥ��������ˮ���������ʣ����ټ���ͭ����ʧ
��������
�ŵ�����̼���������ֹ�������ҪҪ��ֽӴ����ܳ�ַ�Ӧ����ʽ̼��ͭ�����ֽ⣬���ɺ�ɫ������ͭ������ͭ��̼�����Ʒ�Ӧ���ɼ�ʽ̼��ͭ������Ԫ���غ���д��ѧ����ʽ��
�Ƽ�ʽ̼��ͭ����ᷴӦ���ɼ���ͭ������Ԫ���غ���д��ѧ����ʽ������ͭ���ܽ�����¶ȵ����߶��������ȹ��ˣ���������������ԭ������֪����ͭ������ˮ���������л��ܼ���
��a.������̼���������ֹ�������ҪҪ��ֽӴ����ܳ�ַ�Ӧ��������ĥ�������ǰ�ҩƷ��ϸ����Ͼ��ȣ��ʴ�Ϊ����ϸ����Ͼ��ȡ�
b.�¶ȹ��ߣ�Cu(OH)2CuCO3��ֽ����ɺ�ɫ������ͭ���ʴ�Ϊ���к�ɫ�������ɡ�
c.����ͭ��̼�����Ʒ�Ӧ���ɼ�ʽ̼��ͭ������Ԫ���ؿ�֪��ѧ����ʽΪ2CuSO4 + 4NaHCO3 = Cu(OH)2CuCO3�� + 3CO2��+ 2Na2SO4 + H2O���ʴ�Ϊ��2CuSO4 + 4NaHCO3 = Cu(OH)2CuCO3�� + 3CO2��+ 2Na2SO4 + H2O��
��a.��ʽ̼��ͭ����ᷴӦ�Ƶ���ˮ����ͭ[Cu(HCOO)24H2O]���壬����Ԫ���ؿ�֪��ѧ����ʽΪCu(OH)2CuCO3 +4HCOOH + 5H2O =2 Cu(HCOO)24H2O + CO2�����ʴ�Ϊ��Cu(OH)2CuCO3 +4HCOOH + 5H2O =2 Cu(HCOO)24H2O + CO2����
b.����ͭ���ܽ�����¶ȵ����߶���������ȴ�����о������������Ͳ��ʣ��������ȹ��ˣ��ʴ�Ϊ����ֹ����ͭ����������
c.����ͭ������ˮ�������ھƾ�������������ˮϴ�ӣ��������Ҵ�����ϴ�ӣ�ϴȥ��������Һ�����ʣ��ʴ�Ϊ��ϴȥ��������ˮ���������ʣ����ټ���ͭ����ʧ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��������Һ��ֻ���ܺ��Т�Al3+����Mg2+����Fe3+����Fe2+����H+����CO32-����NO3-�еļ��֣������Һ����μ���NaOH��Һ�����������ɳ�����������NaOH�����ʵ����Ĺ�ϵ��ͼ��ʾ�������Һ��һ�����е�������![]()

A.�ݢޢ�B.�ڢۢݢ�C.�٢ڢۢޢ�D.�٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ��Ӧ�Ⱥ�˳���ж���ȷ����![]()
A.���е����ʵ�����![]() ��KOH�Ļ����Һ��ͨ��
��KOH�Ļ����Һ��ͨ��![]() ����
����![]() ��Ӧ�����������ǣ�KOH��
��Ӧ�����������ǣ�KOH�� ![]() ��
��![]()
B.���е����ʵ�����![]() ��
��![]() ��
��![]() �Ļ����Һ�м���Zn����Zn��Ӧ�����������ǣ�
�Ļ����Һ�м���Zn����Zn��Ӧ�����������ǣ�![]() ��
��![]() ��
��![]()
C.���е����ʵ�����![]() ��
��![]() ��
��![]() �Ļ����Һ�еμ����ᣬ�����ᷴӦ�����������ǣ�
�Ļ����Һ�еμ����ᣬ�����ᷴӦ�����������ǣ�![]() ��
��![]() ��
��![]() ��
��![]()
D.�ں��е����ʵ�����![]() ��
��![]() ����Һ�У�����ͨ����������������Ӧ�����������ǣ�
����Һ�У�����ͨ����������������Ӧ�����������ǣ�![]() ��
��![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ա�
��.�����£�ijˮ��ҺM�д��ڵ�������Na����A2����HA����H����OH�������ڵķ�����H2O��H2A����������ش��������⣺
(1)д����H2A�ĵ��뷽��ʽ��
________________________________________________________________________��
(2)ʵ����NaHA��Һ��pH>7�������NaHA��Һ�Լ��Ե�ԭ��
________________________________________________________________________��
��д��NaHA��Һ������Ũ���ɴ�С��˳��
________________________________________________________________________��
��.(1)��ͼ�ױ�ʾ����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ�Ũ����ͬ������һԪ�ᣬ��ͼ��ȷ��������ǿ����________(�����١����ڡ������ۡ�)����ͼ�ұ�ʾ����ͬŨ�ȵ�AgNO3����Һ�ֱ�ζ�Ũ����ͬ�ĺ�Cl����Br����I���Ļ����Һ����ͼ��ȷ�����ȳ�����������________��

(2)25 ��ʱ��Ksp(AgCl)��1.8��10��10����1 L 0.1 mol��L��1 NaCl��Һ�м���1 L 0.2 mol��L��1AgNO3��Һ����ַ�Ӧ����Һ��c(Cl��)��________ mol��L��1(�����Ϻ���Һ������仯���Բ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�ϳ���ȼ�շ��ⶨ�л���ķ���ʽ�����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɡ���ͼ��ʾ������ȼ�շ�ȷ���л������ʽ�ij���װ�á�
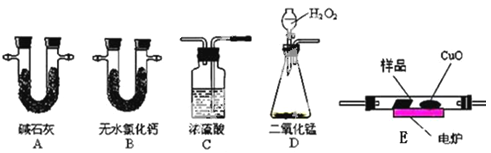
ʵ��̽��С���ȡһ����������Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣�������ʵ��,ͨ���ⶨ������CO2��ˮ������ȷ���л������ɣ���ش��������⣺
��1��C��Ũ����������dz�ȥ�����е�ˮ������ʵ��װ�õ�����˳��Ӧ�ǣ�___________��ÿ��װ��ֻ��һ�Σ���
��2��ʵ�����ݼ�¼�ʹ���
������ʵ����� | ȼ���л�������� | �� | �� | ||
ʵ��ǰ���� | ʵ������� | ʵ��ǰ���� | ʵ������� | ||
1 | m1 | m2 | m3 | m4 | m5 |
�ϱ��Т١��ڷֱ�ָ�ĸ�װ�ã�____________ �� _____________��
��3����ʵ��ȷ��ȡ4.4 g��Ʒ����ȼ�պ��ò���CO28.8 g��ˮ����3.6g��Ҫȷ�����л���ķ���ʽ��������֪����������________��
��4����ͬ�����£������л�����������������Է�������Ϊ22�������ĺ˴Ź����������������壬��ǿ�ȱ�Ϊ3:1����ͨ������ȷ�����л���Ľṹ��ʽ___________������л�����Է���������ͬ������һ�ȴ�����_____�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijpH =1����ҺX�����п��ܺ���Al3+��Fe2+��Fe3+��Ba2+��NH4+��CO32����SO42����SiO32����NO3���е�һ�ֻ��֣�ȡ500 mL����Һ����ʵ�飬������ת����ͼ��ʾ��
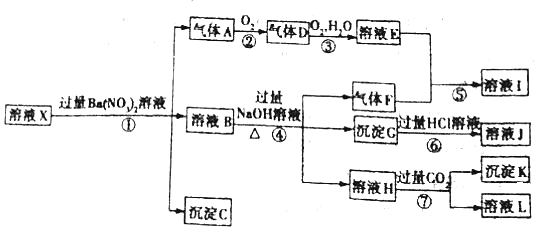
��֪����Ӧ��������һ�������Ǻ���ɫ����ش��������⣺
(1)����ǿ������������ж���ҺX��һ�������ڵ�������______��
(2)��ҺX�У�����NO3�����ж�һ����ȷ����______![]() ����ĸ
����ĸ![]() ��
��
a.һ����
b.һ��û��
c.������
(3)��Ӧ���в�������A�����ӷ���ʽΪ______��
(4)��Ӧ�������ɳ���K�����ӷ���ʽΪ______��
(5)��ҺX�в���ȷ����������______��
(6)��ʵ��ⶨA��F��K��Ϊ0.01 mol����ȷ������C��______�������ʵ�����Χ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���У�������ͷ���������У��ҷ��������������
ʵ��Ŀ�� | ������ | ������ | |
A | ��ȥ���������е��������� | �ӱ��� | ���� |
B | ����̼���ƺ�̼��������Һ | �ֱ�μӳ���ʯ��ˮ | �ֱ��������Һ |
C | �������������Ƿ����� | ���� | ���������ữ�� |
D | �Ƚ���Ԫ�ء���Ԫ�صķǽ�����ǿ�� | �ֱ�����Ȼ��⡢�⻯�⣬�Ƚ����ȶ��� | �ڵ��۵⻯����ֽ�ϵμ���ˮ |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ȩ��һ��ʳƷ���Ӽ�����������ľ����ԭ�Ϻϳɣ��ϳ�·�����£�
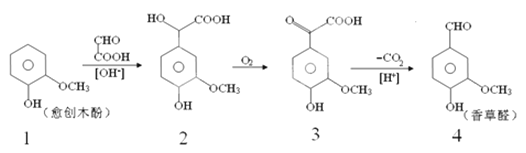
����˵����ȷ���ǣ�������
A. ��Ӧ1��2���ڼӳɷ�Ӧ�������ɵĻ�����2����һ������̼ԭ��
B. ������2��һ�������¿ɷ�����ȥ��Ӧ
C. �����Ƶõ����ȩ���Ƿ���л�����3�������Ȼ�����Һ
D. �����ʵ������ֻ�����ֱ�������NaOH��Ӧ������NaOH���ʵ���֮��Ϊ1��3��2��4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
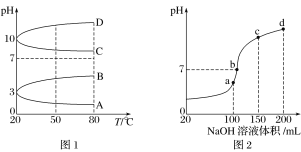
(1)��ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(NH4+)________(������������������������С����)0.1 mol��L��1NH4HSO4��c(NH4+)��
(2)��ͼ1��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(����ĸ)��
������ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)��________mol��L��1(����ֵ����ʽ)��
(3)����ʱ����100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ2��ʾ���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������____________����b�㣬��Һ�и�����Ũ���ɴ�С������˳����______________��
��.pC��ָ��ϡ��Һ���������ʵ���Ũ�ȵij��ö�����ֵ������pH����ij��Һ���ʵ�Ũ��Ϊ1��10��3mol��L��1�������Һ�и����ʵ�pC����lg10��3��3����֪H2CO3��Һ�д�������ƽ�⣺CO2��H2O![]() H2CO3��H2CO3
H2CO3��H2CO3![]() H����HCO3-��HCO3-
H����HCO3-��HCO3-![]() H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺
H����CO32-ͼ3ΪH2CO3��HCO3-��CO32-�ڼ���ǿ���ǿ����Һ�ﵽƽ��ʱ��Һ�����ֳɷֵ�pCpHͼ����ش��������⣺

(1)��pH��9ʱ��H2CO3��Һ��Ũ�����ĺ�̼Ԫ�ص�����Ϊ______��
(2)pH<4ʱ����Һ��H2CO3��pC����Լ����3��ԭ����____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com