
��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��
��1����֪��2Cu(s)��1/2O2(g)=Cu2O(s)����H = -169kJ��mol-1��
C(s)��1/2O2(g)=CO(g)����H = -110.5kJ��mol-1��
Cu(s)��1/2O2(g)=CuO(s)����H = -157kJ��mol-1
��̿���ڸ��������»�ԭCuO����Cu2O���Ȼ�ѧ����ʽ�ǣ�
��2����һ�������£���������������������·�Ӧ��2SO2(g)+O2(g) 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
��3���Լ��顢����Ϊ��Ӧ�KOH��Һ���������Һ����ȼ�ϵ�أ�����ӦʽΪ�� ��
��4����ˮAlCl3ƿ�Ǵ��а������䷴Ӧ�Ļ�ѧ����ʽΪ ��
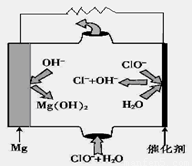
��5����þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ���õ�ط�Ӧ���ܷ�Ӧ����ʽΪ_____________________��
��6����ҵ�ϵ�ⱥ��ʳ��ˮ�����ӷ���ʽΪ________________��
��12�֣���1��2CuO(s)��C(s)= Cu2O(s)��CO(g)����H =+34.5 kJ��mol-1 ��2�֣�
��2��K= c2(SO3)/ c2(SO2) c (O2) ��2�֣�
��3��CH4+10OH����8e��=CO32��+7H2O�� ��2�֣�
��4��AlCl3��3H2O Al(OH)3��3HCl ��2�֣�
Al(OH)3��3HCl ��2�֣�
��5��Mg+ClO-+ H2O= Cl-+Mg(OH)2 ��2�֣�
��6��2Cl����2H2O 2OH����Cl2����H2�� ��2�֣�
2OH����Cl2����H2�� ��2�֣�
��������
�����������1����֪����2Cu��s��+1/2O2��g��=Cu2O��s������H=-169kJ��mol-1��
��C��s��+1/2O2��g��=CO��g������H=-110.5kJ��mol-1��
��Cu��s��+1/2O2��g��=CuO��s������H=-157kJ��mol-1
�ɸ�˹���ɿ�֪����-�ۡ�2+�ڵ�2CuO��s��+C��s��=Cu2O��s��+CO��g������H=-169kJ��mol-1-��-157kJ��mol-1����2=-110.5kJ��mol-1=+34.5 kJ��mol-1��
�ʴ�Ϊ��2CuO��s��+C��s��=Cu2O��s��+CO��g������H=+34.5 kJ��mol-1��
��2�����淴Ӧ2SO2��g��+O2��g�� 2SO3��g���Ļ�ѧƽ�ⳣ��
2SO3��g���Ļ�ѧƽ�ⳣ��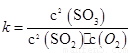
�ʴ�Ϊ��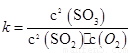
��3��ԭ��ظ�������������Ӧ�������ڸ����ŵ磬������������Ӧ�����������������ŵ��������������ӣ������缫��ӦʽΪ2O2+4H2O+8e��=8OH�����ܵĵ�ط�ӦʽΪCH4+2O2+2OH��=CO32��+3H2O������ܷ�Ӧʽ��ȥ�����缫��Ӧʽ�ɵø����缫��ӦʽΪCH4+10OH��-8e��=CO32��+7H2O��
�ʴ�Ϊ��CH4+10OH��-8e��=CO32��+7H2O��
��4��AlCl3ˮ��AlCl3+3H2O Al��OH��3+3HCl����HCl���Ȼ���������е�ˮ�����ʰ�����
Al��OH��3+3HCl����HCl���Ȼ���������е�ˮ�����ʰ�����
�ʴ�Ϊ��AlCl3+3H2O Al��OH��3+3HCl��
Al��OH��3+3HCl��
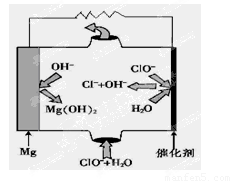
��5����ͼ��֪þ-�������Ρ�ȼ�ϵ����Mg��ClO����H2O��Ӧ����Cl����Mg��OH��2���õ�ط�Ӧ���ܷ�Ӧ����ʽΪMg+ClO��+H2O=Cl��+Mg��OH��2��
�ʴ�Ϊ��Mg+ClO��+H2O=Cl��+Mg��OH��2��
��6����ⱥ��ʳ��ˮ�����������������������ƣ���ⱥ��ʳ��ˮ�����ӷ���ʽΪ2Cl��+2H2O 2OH��+Cl2��+H2����
2OH��+Cl2��+H2����
�ʴ�Ϊ��2Cl��+2H2O 2OH��+Cl2��+H2��
2OH��+Cl2��+H2��
���㣺�ø�˹���ɽ����йط�Ӧ�ȵļ��㣻ԭ��غ͵��صĹ���ԭ������ѧƽ�ⳣ���ĺ���


 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��
��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| c2(SO3) |
| c2(SO2)?c(O2) |
| c2(SO3) |
| c2(SO2)?c(O2) |
 Al��OH��3+3HCl
Al��OH��3+3HCl Al��OH��3+3HCl
Al��OH��3+3HCl
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(4��)��֪25�桢101kpaʱ��һЩ���ʵ�ȼ����Ϊ��
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/(KJ/mol) | -283.0 | -285.8 | -726.5 |
��1��д����������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��2�����ݸ�˹������������Ȼ�ѧ����ʽ CO(g)+ 2H2(g)= CH3OH(l) ��H=
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ������ѧ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
(4��)��֪25�桢101kpaʱ��һЩ���ʵ�ȼ����Ϊ��
| ��ѧʽ | CO(g) | H2(g) | CH3OH(l) |
| ��H/(KJ/mol) | -283.0 | -285.8 | -726.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ���ѡ�ޣ��������棩 ���ͣ������
��������Ȼ�ѧ����ʽ����ѧ����ʽ���缫��Ӧʽ������ʽ�ȣ�����д��
��1����֪��2Cu(s)��1/2O2(g)=Cu2O(s)����H=-169kJ��mol-1��
C(s)��1/2O2(g)=CO(g)����H=-110.5kJ��mol-1��
Cu(s)��1/2O2(g)=CuO(s)����H=-157kJ��mol-1
��̿���ڸ��������»�ԭCuO����Cu2O���Ȼ�ѧ����ʽ�ǣ�
��2����һ�������£���������������������·�Ӧ��2SO2(g)+O2(g) 2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
��3���Լ��顢����Ϊ��Ӧ�KOH��Һ���������Һ����ȼ�ϵ�أ�����ӦʽΪ�� ��
��4�����ڳ�ʪ�Ŀ����з���������ʴ�ĵ�ط�Ӧ����ʽΪ ��
��5����þ���������Ρ�ȼ�ϵ�أ���װ��ʾ��ͼ��ͼ���õ�ط�Ӧ���ܷ�Ӧ����ʽΪ_______________ ��
��6����ҵ�ϵ�������Ȼ��Ƶķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�긣��ʡ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
(4��)��֪25�桢101kpaʱ��һЩ���ʵ�ȼ����Ϊ��
|
��ѧʽ |
CO(g) |
H2(g) |
CH3OH(l) |
|
��H/(KJ/mol) |
-283.0 |
-285.8 |
-726.5 |
��1��д����������CH3OH(l)��ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��2�����ݸ�˹������������Ȼ�ѧ����ʽ CO(g)+ 2H2(g)= CH3OH(l) ��H=
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com