
50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺

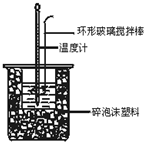
��1����ʵ��װ�ÿ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��_______________��
��2���ձ���������ֽ����������_________________________��
��3��ʵ���и���60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����Ƚϣ����ų�������__________�����ȡ�����ȡ������к���__________�����ȡ�����ȡ�����������__________________________________��
��4����50mL0.50mol/L�Ĵ������������Һ��������ʵ�飬����к��ȵ���ֵ��57.3kJ/mol��Ƚϻ�____________�����ƫ����ƫС������Ӱ�족����
��5����������һ��������ʵ�飬��ⶨ______���¶ȡ�
��6�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (�ƫ����ƫС������Ӱ�족)
��1�����β�������� ��2������ʵ�������������ʧ
��3������� ��� ��Ϊ�к�����ָ��ϡ��Һ�У��������кͷ�Ӧ����1molH2O���ų�������������������� ��ƫС ��5��3 ��6��ƫС
�������������к��ȵIJⶨ����������
��1����ʵ������У���ʹ��Һ��Ͼ��ȣ���Ҫ���裬��˺�ȱ�ٵ������ǻ��β����������
��2����ʵ������У���Ҫ�����ܵļ�����������ʧ�������ձ���������ֽ���������Ǽ���ʵ�������������ʧ��
��3�������к�����ָ��ϡ��Һ�У��������кͷ�Ӧ����1molH2O���ų�������������������ء����Ըı��������������Ӧ�зų����������Ըı䣬���к����Dz���ġ�
��4��������������ʣ����ڵ���ƽ�⡣�����������ȵģ����Բⶨ����ֵ��ƫС��
��5��Ϊ�˼�Сʵ����Ӧ�����ٲⶨ3���¶ȣ�Ȼ������ƽ��ֵ��
��6�����ձ����粻��Ӳֽ�壬����������ʧ����˵õ��к�����ֵƫС��


 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵������ȷ���ǣ�������
�к��Ȳⶨʵ���У���50mL0.50mol/L�����50mL0.55mol/LNaOH����ʵ�飬����˵������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺
50ml0.50mol?L-1������50mL0.55mol?L-1NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȣ��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ǰ���� | ����ǰ���� | ���Ⱥ����� |
| W1�������� | W2������+���壩 | W3������+��ˮ����ͭ�� |
| 160(W2-W3) |
| 18(W3-W1) |
| 160(W2-W3) |
| 18(W3-W1) |
| 0.418��2.35 |
| 0.025 |
| 0.418��2.35 |
| 0.025 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com