
Ä³Ń§ÉśÓƵē×ÓĢģĘ½ŗĶ±ŲŅŖµÄ»ÆѧŹµŃéŅĒĘ÷²ā¶ØĢśĀĮŗĻ½šÖŠø÷×é·Öŗ¬Į棬Ę䏵Ńé×°ÖĆČēĶ¼ĖłŹ¾£®ŹµŃ鏱£¬Č”Ņ»æéĢśĀĮŗĻ½š£¬½«ĘäĒŠ³ÉĖéæéŗóČ«²æ¼ÓČėµ½Ź¢ÓŠ50 mL””5.0 mol”¤L£1””NaOHČÜŅŗµÄÉÕ±ÖŠ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŗĻ½š±ķĆę×īĻČ²śÉśĘųÅŻµÄĪ»ÖĆŹĒ________(Ģī”°ĒŠæŚ¶ĻĆę”±»ņ”°·ĒĒŠæŚ¶ĻĆę”±)£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________£®
(2)²»Ķ¬Ź±¼äµē×ÓĢģĘ½µÄ¶ĮŹżČēĻĀ±ķĖłŹ¾£ŗ
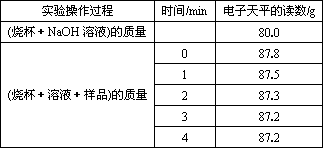
ŌņŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹżĪŖ________(±£ĮōČżĪ»ÓŠŠ§Źż×Ö£¬ĻĀĶ¬)£®
(3)ÉĻŹö50 mL””NaOHČÜŅŗÖŠ×ī¶ąæɼÓČėµÄĢśĀĮŗĻ½šŃłĘ·µÄÖŹĮæĪŖ________£®
|
””””“š°ø£ŗ(1)ĒŠæŚ¶ĻĆę£»2Al£«2OH££«2H2O£½2AlO ””””½āĪö£ŗ(1)Ģś”¢ĀĮ»ÆѧŠŌÖŹ½Ļ»īĘĆ£¬±ķĆę¾łŅ×Éś³ÉŃõ»ÆĪļ±”Ĥ£¬¹ŹŌŚ”°ĒŠæŚ¶ĻĆę”±×īĻČ²śÉśĘųÅŻ£¬ĒŅAl²śÉśAl3+ŗó£¬ÓÉÓŚNaOH¹żĮ棬¹ŹĄė×Ó·½³ĢŹ½Ó¦æ¼ĀĒAl(OH)3ÓėNaOHµÄ·“Ó¦£® ””””(2)ŗĻ½šÖŠÖ»ÓŠAlÓėNaOHČÜŅŗ·“Ó¦£¬ĒŅČÜŅŗ¼õÉŁµÄÖŹĮæŹĒH2µÄÖŹĮ棬Ōņ£ŗ
|


 ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ÓƶžŃõ»ÆĀČ£ØClO2£©”¢øßĢśĖįÄĘ£ØNa2FeO4Ħ¶ūÖŹĮæĪŖ166g?mol-1£©µČŠĀŠĶ¾»Ė®¼ĮĢę“ś“«Ķ³µÄ¾»Ė®¼ĮCl2¶ŌµĖ®½ųŠŠĻū¶¾ŹĒ³ĒŹŠŅūÓĆĖ®“¦ĄķŠĀ¼¼Źõ£®ClO2ŗĶNa2FeO4ŌŚĖ®“¦Ąķ¹ż³ĢÖŠ·Ö±š±»»¹ŌĪŖCl-ŗĶFe3+£®
ÓƶžŃõ»ÆĀČ£ØClO2£©”¢øßĢśĖįÄĘ£ØNa2FeO4Ħ¶ūÖŹĮæĪŖ166g?mol-1£©µČŠĀŠĶ¾»Ė®¼ĮĢę“ś“«Ķ³µÄ¾»Ė®¼ĮCl2¶ŌµĖ®½ųŠŠĻū¶¾ŹĒ³ĒŹŠŅūÓĆĖ®“¦ĄķŠĀ¼¼Źõ£®ClO2ŗĶNa2FeO4ŌŚĖ®“¦Ąķ¹ż³ĢÖŠ·Ö±š±»»¹ŌĪŖCl-ŗĶFe3+£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
![]()
£Ø1£©ÓĆĶŠÅĢĢģĘ½ŗĶŠ”ÉÕ±³Ę³öŅ»Ņ©³×ѳʷµÄÖŹĮ棬ĘäÕżČ·²Ł×÷µÄĖ³ŠņĪŖ____________”£
¢Łµ÷Įćµć£¬µ÷½ŚĢģĘ½×óÓŅµÄŠ”ĀŻĖ棬Ź¹ÖøÕė¾²Ö¹Ź±øÕŗĆ“¦ÓŚ±ź³ßæĢ¶ČÅĢĮ擦¢Ś½«ÓĪĀė²¦·ÅŌŚæĢ¶Č³ß×ī×ó¶ĖĮ擦¢Ū³ĘĮææյĊ”ÉÕ±¢ÜȔѳʷ·ÅČėŠ”ÉÕ±ŗóŌŁ³ĘĮæ¢Ż½«ķĄĀė·Å»ŲķĄĀėŗŠ
£Ø2£©ČܽāѳʷĖłŠčŅĒĘ÷ÓŠ____________”£
£Ø3£©¼ÓČėµÄAČÜŅŗŹĒ____________£¬¼Ó¹żĮæŹŌ¼ĮµÄÄæµÄŹĒ____________£¬Č·ČĻB¹żĮæµÄ·½·ØŹĒ____________”£
£Ø4£©¹żĀĖŹ±£¬Ä³Ń§ÉśÓĆČēĻĀĶ¼ĖłŹ¾µÄ²Ł×÷£¬Ęä“ķĪó“¦ÓŠ£ŗ¢Ł____________£»¢Ś____________”£
£Ø5£©³ĮµķŠčĻ“µÓµÄŌŅņŹĒ______________£¬Ļ“µÓ·½·ØĪŖ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
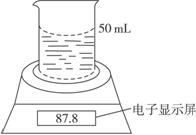
ŹµŃ鏱£¬Č”Ņ»æéĢśĀĮŗĻ½š£¬½«ĘäĒŠ³ÉĖéæéŗóČ«²æ¼ÓČėµ½Ź¢ÓŠ50 mL 5.0 mol”¤L-1NaOHČÜŅŗµÄÉÕ±ÖŠ£¬ŹŌĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŗĻ½š±ķĆę×īĻČ²śÉśĘųÅŻµÄĪ»ÖĆŹĒ_____________£ØĢī”°ĒŠæŚ¶ĻĆę”±»ņ”°·ĒĒŠæŚ¶ĻĆę”±£©£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ________________________________________________________”£
£Ø2£©ŗĻ½š±ķĆę»įŠĪ³ÉŌµē³Ų£¬Ęäøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗ______________________________”£
£Ø3£©²»Ķ¬Ź±¼äµē×ÓĢģĘ½µÄ¶ĮŹżČēĻĀ±ķĖłŹ¾£ŗ
ŹµŃé²Ł×÷¹ż³Ģ | Ź±¼ä/min | µē×ÓĢģĘ½µÄ¶ĮŹż/g |
ÉÕ±+NaOHČÜŅŗ |
| 80.0 |
ÉÕ±+ČÜŅŗ+ѳʷ | 0 | 87.8 |
1 | 87.5 | |
2 | 87.3 | |
3 | 87.2 | |
4 | 87.2 |
ŌņŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹżĪŖ£Ø±£ĮōČżĪ»ÓŠŠ§Źż×Ö£¬ĻĀĶ¬£©”£
£Ø4£©ÉĻŹö50 mL NaOHČÜŅŗÖŠ×ī¶ąæɼÓČėµÄĢśĀĮŗĻ½šŃłĘ·ÖŹĮæĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

ŹµŃ鏱£¬Č”Ņ»æéĢśĀĮŗĻ½š£¬½«ĘäĒŠ³ÉĖéæéŗóČ«²æ¼ÓČėµ½Ź¢ÓŠ50 mL 5.0 mol”¤L-1 NaOHČÜŅŗµÄÉÕ±ÖŠ”£ŹŌĶź³ÉĻĀĮŠĪŹĢā£ŗ
(1)ŗĻ½š±ķĆę×īĻČ²śÉśĘųÅŻµÄĪ»ÖĆŹĒ___________ (Ģī”°ĒŠæŚ¶ĻĆę”±»ņ”°·ĒĒŠæŚ¶ĻĆę”±)£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ__________________________________”£
(2)ŗĻ½š±ķĆę»įŠĪ³ÉŌµē³Ų£¬Ęäøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗ__________________________________”£
(3)²»Ķ¬Ź±¼äµē×ÓĢģĘ½µÄ¶ĮŹżČēĻĀ±ķĖłŹ¾£ŗ
ŹµŃé²Ł×÷¹ż³Ģ | Ź±¼ä/min | µē×ÓĢģĘ½µÄ¶ĮŹż/g |
ÉÕ±+NaOHČÜŅŗ |
| 80.0 |
ÉÕ±+ČÜŅŗ+ѳʷ | 0 | 87.8 |
1 | 87.5 | |
2 | 87.3 | |
3 | 87.2 | |
4 | 87.2 |
ŌņŗĻ½šÖŠĢśµÄÖŹĮæ·ÖŹżĪŖ____________(±£ĮōČżĪ»ÓŠŠ§Źż×Ö£¬ĻĀĶ¬)”£
(4)ÉĻŹö50 mL NaOHČÜŅŗÖŠ×ī¶ąæɼÓČėµÄĢśĀĮŗĻ½šŃłĘ·ÖŹĮæĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com