



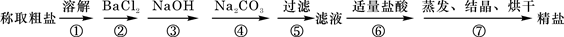
 һ����������ϵ�д�
һ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ӵ�ˮ����ȡ�⣺�þƾ���ȡ |
| B����ȥCO������O2��ͨ�����ȵ�Cu�����ռ����� |
| C����ȥKCl��Һ�е�����MgCl2����������NaOH��Һ������ |
| D����ȥCO2�е�����HCl��ͨ�뱥��NaHCO3��Һ���ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����Һ | B������ | C���ᾧ | D������ E������ F������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ij��Һ��NaOH��Һ���ȣ�������ʹʪ�����ɫʯ����ֽ�������壬˵��ԭ��Һ�д���笠����� |
| B��ij��Һ�м�����������Һʱ��������ɫ��������ϡ����������ܽ⣬˵��ԭ��Һ�к�Cl- |
| C���ò�˿պȡij��Һ�ھƾ��ƵĻ���������ʱ������ʻ�ɫ��˵��ԭ��Һ��ֻ����Na+�� ������K+�� |
| D��ij��Һ�м���BaCl2��Һʱ��������ɫ��������ϡ����������ܽ⣬˵��ԭ��Һ�д���SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥKNO3�е�NaCl����-------�ᾧ���ؽᾧ | B����ȥʳ���е���ɳ---�ܽ⡢���� |
| C����ȥKCl�е�K2CO3--------���������� | D����ȥ��ˮ�е�ˮ-------��CCl4��ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ù����ļ�Һ��ȥCO2�л��е�����HCl���� |
| B���ñ���ʳ��ˮ��ȥ�����л��е�SO2���� |
| C���������ķ�����ȥʳ����������I2 |
| D���ù������Ȼ�����Һ��ȥ����������Һ�е�����SO42�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com