
ĻÖÓŠĻĀĮŠæÉÄę·“Ó¦£ŗA(g)£«B(g)  xC(g)£¬ŌŚ²»Ķ¬Ģõ¼žĻĀÉś³ÉĪļCŌŚ·“Ó¦»ģŗĻĪļÖŠµÄÖŹĮæ·ÖŹż(C%)ŗĶ·“Ó¦Ź±¼ä(t)µÄ¹ŲĻµČēĻĀĶ¼£ŗ
xC(g)£¬ŌŚ²»Ķ¬Ģõ¼žĻĀÉś³ÉĪļCŌŚ·“Ó¦»ģŗĻĪļÖŠµÄÖŹĮæ·ÖŹż(C%)ŗĶ·“Ó¦Ź±¼ä(t)µÄ¹ŲĻµČēĻĀĶ¼£ŗ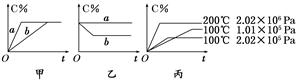
Ēėøł¾ŻĶ¼Ļń»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Čō¼×Ķ¼ÖŠĮ½ĢõĒśĻß·Ö±š±ķŹ¾ÓŠ“߻ƼĮŗĶĪŽ“߻ƼĮµÄĒéæö£¬Ōņ ĒśĻß±ķŹ¾ĪŽ“߻ƼĮŹ±µÄĒéæö(Ģī×ÖÄø£¬ĻĀĶ¬)£»
(2)ČōŅŅĶ¼±ķŹ¾·“Ó¦“ļµ½Ę½ŗāŗó·Ö±šŌŚŗćĪĀŗćŃ¹Ģõ¼žĻĀŗĶŗćĪĀŗćČŻĢõ¼žĻĀĻņĘ½ŗā»ģŗĻĘųĢåÖŠ³äČė¶čŠŌ(Óė·“Ó¦ĢåĻµÖŠČĪŅ»ĪļÖŹ¾ł²»·“Ó¦)ĘųĢåŗóµÄĒéæö£¬Ōņ ĒśĻß±ķŹ¾ŗćĪĀŗćČŻµÄĒéæö£»
(3)øł¾Ż±ūĶ¼æÉŅŌÅŠ¶ĻøĆæÉÄę·“Ó¦µÄÕż·“Ó¦ŹĒ ČČ·“Ó¦(Ģī”°Īü”±»ņ”°·Å”±)£»
(4)»Æѧ¼ĘĮæŹżxµÄÖµ (ĢīȔֵ·¶Ī§)£»ÅŠ¶ĻµÄŅĄ¾ŻŹĒ ”£
 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø17·Ö£©H2O2ŹĒŅ»ÖÖĒæŃõ»Æ¼Į£¬±»¹ć·ŗÓ¦ÓĆÓŚĖ®“¦Ąķ¼°ĪĄÉśĻū¶¾µČ·½Ćę”£
£Ø1£©H2O2²»ĪČ¶Ø£¬µ±ĘäÖŠŗ¬Fe2£«Ź±£¬»į·¢Éś·“Ó¦£ŗ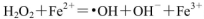
 £¬ŌņFe2£«ŌŚ“Ė¹ż³ĢÖŠĖłĘšµÄ×÷ÓĆŹĒ______________£¬µ±Éś³É336mL O2£Ø±ź×¼×“æö£©Ź±£¬·“Ó¦ÖŠ×ŖŅʵē×ÓµÄĪļÖŹµÄĮæĪŖ_______mol”£
£¬ŌņFe2£«ŌŚ“Ė¹ż³ĢÖŠĖłĘšµÄ×÷ÓĆŹĒ______________£¬µ±Éś³É336mL O2£Ø±ź×¼×“æö£©Ź±£¬·“Ó¦ÖŠ×ŖŅʵē×ÓµÄĪļÖŹµÄĮæĪŖ_______mol”£
£Ø2£©ĻĀ±ķŹĒŌŚ³£Ń¹”¢60”ęŗĶ²»Ķ¬pHĢõ¼žĻĀ£¬6mL30% H2O2ŌŚ60minÄŚŹĶ·Å³öŃõĘųµÄĢå»ż”£ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ___________”£
a£®pHŌ½“ó£¬H2O2µÄ·Ö½āĖŁĀŹŌ½“ó
b£®pHŌŚ9×óÓŅ£¬H2O2µÄ·Ö½āĖŁĀŹ×ī“ó
c£®6mL 30% H2O2·Ö½ā×ī¶ąŹĶ·Å³öµÄŃõĘųµÄĢå»żĪŖ639mL
d. pH=5.50Ź±£¬0”«60minÄŚ£¬v(O2)=1.5mL
£Ø3£©ČÜŅŗÖŠH2O2µÄ²ŠĮōĮææÉÓĆŅ»¶ØÅØ¶ČµÄĖįŠŌKMnO4ČÜŅŗĄ“²ā¶Ø£¬·“Ó¦ÖŠMnO4£
±»»¹ŌĪŖMn2£«£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________”£
£Ø4£©æĘѧ¹¤×÷ÕßŅŌIr-Ru/TiĪŖŃō¼«”¢ACFCĪŖŅõ¼«£¬ŌŚĖįŠŌ»·¾³”¢²»¶ĻĶØČėæÕĘųµÄĢõ¼žĻĀÖ±½Óµē½āĖ®Ą“ÖʱøH2O2”£µē½ā¹ż³ĢÖŠ£¬Ńō¼«ĒųČÜŅŗµÄpH_ £ØĢī”°Ōö“ó”±”°²»±ä”±»ņ”°¼õŠ””±£©£¬Ņõ¼«²śÉśH2O2µÄµē¼«·“Ó¦Ź½ĪŖ_______”£Čō²»ĶØæÕĘų£¬ŌņŅõ¼«µĆµ½µÄ²śĪļŹĒ_______ ”£
£Ø5£©¼ŗÖŖ¶ĻĮŃ1mol»Æѧ¼üĖłŠčµÄÄÜĮæ
143,H-OĪŖ463”£Ōņ .
.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ęū³µĪ²ĘųÖŠŗ¬ÓŠCO”¢NO2µČÓŠ¶¾ĘųĢ壬¶ŌĘū³µ¼Ó×°Ī²Ęų¾»»Æ×°ÖĆ£¬æÉŹ¹ÓŠ¶¾ĘųĢåĻą»„·“Ó¦×Ŗ»ÆĪŖĪŽ¶¾ĘųĢ唣Ęū³µĪ²ĘųÖŠCOÓėH2O(g)ŌŚŅ»¶ØĢõ¼žĻĀæÉŅŌ·¢Éś·“Ó¦£ŗ
CO(g)£«H2O(g) CO2(g)£«H2(g)¦¤H£¼0”£820 ”ꏱŌŚ¼×”¢ŅŅ”¢±ūČżøöŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±°“ÕÕĻĀ±ķ½ųŠŠĶ¶ĮĻ£¬“ļµ½Ę½ŗāדĢ¬£¬K£½1.0”£
CO2(g)£«H2(g)¦¤H£¼0”£820 ”ꏱŌŚ¼×”¢ŅŅ”¢±ūČżøöŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±°“ÕÕĻĀ±ķ½ųŠŠĶ¶ĮĻ£¬“ļµ½Ę½ŗāדĢ¬£¬K£½1.0”£
| ĘšŹ¼ĪļÖŹµÄĮæ | ¼× | ŅŅ | ±ū |
| n(H2O)/mol | 0.10 | 0.20 | 0.20 |
| n(CO)/mol | 0.10 | 0.10 | 0.20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø18·Ö£©ŅŃÖŖæÉÄę·“Ó¦CO2(g) + H2(g) CO(g) + H2O(g)£¬
CO(g) + H2O(g)£¬
¢ÅŠ“³öøĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½£ŗK= ”£
¢Ę830KŹ±£¬ČōĘšŹ¼Ź±£ŗc (CO2)=2mol/L£¬c (H2)=3mol/L£¬Ę½ŗāŹ±CO2µÄ×Ŗ»ÆĀŹĪŖ60%£¬ĒāĘųµÄ×Ŗ»ÆĀŹĪŖ £»KÖµĪŖ ”£
¢Ē830KŹ±£¬ČōÖ»½«ĘšŹ¼Ź±c (H2)øÄĪŖ6mol/L£¬ŌņĒāĘųµÄ×Ŗ»ÆĀŹĪŖ ”£
¢ČČō830KŹ±£¬ĘšŹ¼ÅضČc (CO2) =" a" mol/L£¬c (H2) =" b" mol/L£¬H2OµÄĘ½ŗāÅضČĪŖc (H2O) =" c" mol/L£¬Ōņ£ŗ¢Ła”¢b”¢cÖ®¼äµÄ¹ŲĻµŹ½ŹĒ £»¢Śµ±a = bŹ±£¬a = c”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
·“Ó¦A(g)  B(g) +C(g)ŌŚČŻ»żĪŖ1.0LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ£¬AµÄ³õŹ¼ÅضČĪŖ0.050mol/L”£ĪĀ¶ČT1ŗĶT2ĻĀAµÄÅضČÓėŹ±¼ä¹ŲĻµČēĶ¼ĖłŹ¾”£»Ų“šĻĀĮŠĪŹĢā£ŗ
B(g) +C(g)ŌŚČŻ»żĪŖ1.0LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠ£¬AµÄ³õŹ¼ÅضČĪŖ0.050mol/L”£ĪĀ¶ČT1ŗĶT2ĻĀAµÄÅضČÓėŹ±¼ä¹ŲĻµČēĶ¼ĖłŹ¾”£»Ų“šĻĀĮŠĪŹĢā£ŗ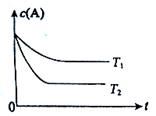
£Ø1£©ÉĻŹö·“Ó¦µÄĪĀ¶ČT1 T2£¬Ę½ŗā³£ŹżK£ØT1£© K£ØT2£©”££ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©
£Ø2£©ČōĪĀ¶ČT2Ź±£¬5minŗó·“Ó¦“ļµ½Ę½ŗā£¬AµÄ×Ŗ»ÆĀŹĪŖ70%£¬Ōņ£ŗ
¢ŁĘ½ŗāŹ±ĢåĻµ×ܵÄĪļÖŹµÄĮæĪŖ ”£
¢Ś·“Ó¦µÄĘ½ŗā³£ŹżK= ”£
¢Ū·“Ó¦ŌŚ0~5minĒų¼äµÄĘ½¾ł·“Ó¦ĖŁĀŹv(A)= ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»·¾³ÖŠ³£¼ūµÄÖŲ½šŹōĪŪČ¾ĪļÓŠ£ŗ¹Æ”¢Ē¦”¢ĆĢ”¢øõ”¢ļÓ”£“¦Ąķ¹¤Ņµ·ĻĖ®ÖŠŗ¬ÓŠµÄCr2O72-ŗĶCrO42-£¬³£ÓƵķ½·ØĪŖ»¹Ō³Įµķ·Ø£¬øĆ·ØµÄ¹¤ŅÕĮ÷³ĢĪŖCrO42-H£«¢Ł×Ŗ»ÆCr2O72-Fe2£«¢Ś»¹ŌCr3£«OH£¢Ū³ĮµķCr(OH)3”ż”£
ĘäÖŠµŚ¢Ł²½“ęŌŚĘ½ŗā2CrO42- (»ĘÉ«)£«2H£« Cr2O72- (³ČÉ«)£«H2O”£
Cr2O72- (³ČÉ«)£«H2O”£
(1)Š“³öµŚ¢Ł²½·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ ”£
(2)¹ŲÓŚµŚ¢Ł²½·“Ó¦£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£
A£®Ķعż²ā¶ØČÜŅŗµÄpHæÉŅŌÅŠ¶Ļ·“Ó¦ŹĒ·ńŅŃ“ļµ½Ę½ŗāדĢ¬
B£®øĆ·“Ó¦ĪŖŃõ»Æ»¹Ō·“Ó¦
C£®ĒæĖįŠŌ»·¾³£¬ČÜŅŗµÄŃÕÉ«ĪŖ³ČÉ«
(3)µŚ¢Ś²½ÖŠ£¬»¹Ō0.1 mol Cr2O72-£¬ŠčŅŖ molµÄFeSO4”¤7H2O”£
(4)µŚ¢Ū²½³żÉś³ÉCr(OH)3Ķā£¬»¹æÉÄÜÉś³ÉµÄ³ĮµķĪŖ ”£
(5)ŌŚČÜŅŗÖŠ“ęŌŚŅŌĻĀ³ĮµķČܽāĘ½ŗā£ŗCr(OH)3(s)  Cr3£«(aq)£«3OH£(aq)£¬³£ĪĀĻĀ£¬Cr(OH)3µÄČܶȻżKsp£½10£32£¬µ±c(Cr3£«)½µÖĮ10£5 mol/L£¬ČĻĪŖc(Cr3£«)ŅŃ¾ĶźČ«³Įµķ£¬ĻÖ½«µŚ¢Ū²½ČÜŅŗµÄpHµ÷ÖĮ4£¬ĒėĶعż¼ĘĖćĖµĆ÷Cr3£«ŹĒ·ń³ĮµķĶźČ«(ĒėŠ“³ö¼ĘĖć¹ż³Ģ)£ŗ ”£
Cr3£«(aq)£«3OH£(aq)£¬³£ĪĀĻĀ£¬Cr(OH)3µÄČܶȻżKsp£½10£32£¬µ±c(Cr3£«)½µÖĮ10£5 mol/L£¬ČĻĪŖc(Cr3£«)ŅŃ¾ĶźČ«³Įµķ£¬ĻÖ½«µŚ¢Ū²½ČÜŅŗµÄpHµ÷ÖĮ4£¬ĒėĶعż¼ĘĖćĖµĆ÷Cr3£«ŹĒ·ń³ĮµķĶźČ«(ĒėŠ“³ö¼ĘĖć¹ż³Ģ)£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
»·¾³ÖŠ³£¼ūµÄÖŲ½šŹōĪŪČ¾ĪļÓŠ£ŗ¹Æ.Ē¦.ĆĢ.øõ.ļÓ”£“¦Ąķ¹¤Ņµ·ĻĖ®ÖŠŗ¬ÓŠµÄ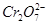 ŗĶ
ŗĶ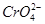 £¬³£ÓƵķ½·ØÓŠĮ½ÖÖ”£
£¬³£ÓƵķ½·ØÓŠĮ½ÖÖ”£
·½·Ø1””»¹Ō³Įµķ·ØøĆ·ØµÄ¹¤ŅÕĮ÷³ĢĪŖ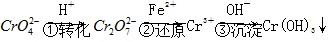 ”£
ӣ
ĘäÖŠµŚ¢Ł²½“ęŌŚĘ½ŗā2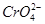 (»ĘÉ«)£«2H£«
(»ĘÉ«)£«2H£«
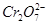 (³ČÉ«)£«H2O”£
(³ČÉ«)£«H2O”£
(1)Š“³öµŚ¢Ł²½·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½_________________________________”£
(2)¹ŲÓŚµŚ¢Ł²½·“Ó¦£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£
A£®Ķعż²ā¶ØČÜŅŗµÄpHæÉŅŌÅŠ¶Ļ·“Ó¦ŹĒ·ńŅŃ“ļĘ½ŗāדĢ¬
B£®øĆ·“Ó¦ĪŖŃõ»Æ»¹Ō·“Ó¦
C£®ĒæĖįŠŌ»·¾³£¬ČÜŅŗµÄŃÕÉ«ĪŖ³ČÉ«
(3)µŚ¢Ś²½ÖŠ£¬»¹Ō0.1 mol 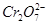 £¬ŠčŅŖ________molµÄFeSO4”¤7H2O”£
£¬ŠčŅŖ________molµÄFeSO4”¤7H2O”£
(4)µŚ¢Ū²½³żÉś³ÉCr(OH)3Ķā£¬»¹æÉÄÜÉś³ÉµÄ³ĮµķĪŖ________”£ŌŚČÜŅŗÖŠ“ęŌŚŅŌĻĀ³ĮµķČܽāĘ½ŗā£ŗCr(OH)3(s)  Cr3£«(aq)£«3OH£(aq)£¬³£ĪĀĻĀ£¬Cr(OH)3µÄČܶȻżKsp£½10£32£¬µ±c(Cr3£«)½µÖĮ10£5 mol”¤L£1Ź±£¬ČĻĪŖc(Cr3£«)ŅŃ¾ĶźČ«³Įµķ£¬ĻÖ½«µŚ¢Ū²½ČÜŅŗµÄpHµ÷ÖĮ4£¬ĒėĶعż¼ĘĖćĖµĆ÷Cr3£«ŹĒ·ń³ĮµķĶźČ«(ĒėŠ“³ö¼ĘĖć¹ż³Ģ)£ŗ______________________________________________”£
Cr3£«(aq)£«3OH£(aq)£¬³£ĪĀĻĀ£¬Cr(OH)3µÄČܶȻżKsp£½10£32£¬µ±c(Cr3£«)½µÖĮ10£5 mol”¤L£1Ź±£¬ČĻĪŖc(Cr3£«)ŅŃ¾ĶźČ«³Įµķ£¬ĻÖ½«µŚ¢Ū²½ČÜŅŗµÄpHµ÷ÖĮ4£¬ĒėĶعż¼ĘĖćĖµĆ÷Cr3£«ŹĒ·ń³ĮµķĶźČ«(ĒėŠ“³ö¼ĘĖć¹ż³Ģ)£ŗ______________________________________________”£
·½·Ø2””µē½ā·Ø
(5)ŹµŃéŹŅĄūÓĆČēĶ¼×°ÖĆÄ£Äāµē½ā·Ø“¦Ąķŗ¬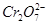 µÄ·ĻĖ®£¬µē½āŹ±Ńō¼«·“Ó¦Ź½ĪŖ________£¬Ņõ¼«·“Ó¦Ź½ĪŖ________£¬µĆµ½µÄ½šŹōŃōĄė×ÓŌŚŅõ¼«ĒųæɳĮµķĶźČ«£¬“ÓĖ®µÄµēĄėĘ½ŗā½Ē¶Č½āŹĶĘäŌŅņŹĒ________________________”£
µÄ·ĻĖ®£¬µē½āŹ±Ńō¼«·“Ó¦Ź½ĪŖ________£¬Ņõ¼«·“Ó¦Ź½ĪŖ________£¬µĆµ½µÄ½šŹōŃōĄė×ÓŌŚŅõ¼«ĒųæɳĮµķĶźČ«£¬“ÓĖ®µÄµēĄėĘ½ŗā½Ē¶Č½āŹĶĘäŌŅņŹĒ________________________”£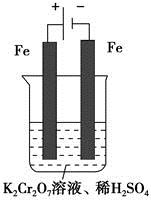
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖNO2ŗĶN2O4æÉŅŌĻą»„×Ŗ»Æ£ŗ2NO2(g)  N2O4(g)(Õż·“Ó¦ĪŖ·ÅČČ·“Ó¦)”£ĻÖ½«Ņ»¶Ø
N2O4(g)(Õż·“Ó¦ĪŖ·ÅČČ·“Ó¦)”£ĻÖ½«Ņ»¶Ø
ĮæNO2ŗĶN2O4µÄ»ģŗĻĘųĢåĶØČėŅ»Ģå»żĪŖ1 LµÄŗćĪĀĆܱÕČŻĘ÷ÖŠ£¬·“Ó¦ĪļÅضČĖꏱ¼ä±ä»Æ¹ŲĻµČēĶ¼ĖłŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)Ķ¼ÖŠ¹²ÓŠĮ½ĢõĒśĻßXŗĶY£¬ĘäÖŠĒśĻß________±ķŹ¾NO2ÅضČĖꏱ¼äµÄ±ä»Æ£»a”¢b”¢c”¢dĖÄøöµćÖŠ£¬±ķŹ¾»Æѧ·“Ó¦“¦ÓŚĘ½ŗāדĢ¬µÄµćŹĒ________”£
(2)Ē°10 minÄŚÓĆNO2±ķŹ¾µÄ»Æѧ·“Ó¦ĖŁĀŹv(NO2)£½________mol/(L”¤min)£»·“Ó¦½ųŠŠÖĮ25 minŹ±£¬ĒśĻß·¢Éś±ä»ÆµÄŌŅņŹĒ________”£
(3)ČōŅŖ“ļµ½Óė×īŗóĻąĶ¬µÄ»ÆŃ§Ę½ŗāדĢ¬£¬ŌŚ25 minŹ±»¹æÉŅŌ²ÉČ”µÄ“ėŹ©ŹĒ________”£
| A£®¼ÓČė“߻ƼĮ | B£®ĖõŠ”ČŻĘ÷Ģå»ż |
| C£®ÉżøßĪĀ¶Č | D£®¼ÓČėŅ»¶ØĮæµÄN2O4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚŅ»¶ØĢõ¼žĻĀ£¬¶žŃõ»ÆĮņŗĶŃõĘų·¢ÉśČēĻĀ·“Ó¦£ŗ2SO2£Øg£©+O2£Øg£©?2SO3£Øg£©£Ø”÷H£¼0£©
£Ø1£©Š“³öøĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½K=
£Ø2£©½µµĶĪĀ¶Č£¬øĆ·“Ó¦KÖµ £¬¶žŃõ»ÆĮņ×Ŗ»ÆĀŹ £¬»Æѧ·“Ó¦ĖŁĀŹ £ØŅŌÉĻ¾łĢīŌö“󔢼õŠ”»ņ²»±ä£©”£
£Ø3£©600”ꏱ£¬ŌŚŅ»ĆܱÕČŻĘ÷ÖŠ£¬½«¶žŃõ»ÆĮņŗĶŃõĘų»ģŗĻ£¬·“Ó¦¹ż³ĢÖŠSO2”¢O2”¢SO3ĪļÖŹµÄĮæ±ä»ÆČēĶ¼£¬·“Ó¦“¦ÓŚĘ½ŗāדĢ¬µÄŹ±¼äŹĒ ”£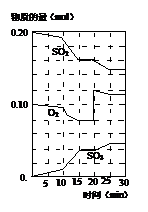
£Ø4£©¾ŻĶ¼ÅŠ¶Ļ£¬·“Ó¦½ųŠŠÖĮ20minŹ±£¬ĒśĻß·¢Éś±ä»ÆµÄŌŅņŹĒ £ØÓĆĪÄ×Ö±ķ“ļ£©”£
£Ø5£©10minµ½15minµÄĒśĻß±ä»ÆµÄŌŅņæÉÄÜŹĒ £ØĢīŠ“±ąŗÅ£©”£
a£®¼ÓĮĖ“߻ƼĮ b£®ĖõŠ”ČŻĘ÷Ģå»ż
c£®½µµĶĪĀ¶Č d£®Ōö¼ÓSO3µÄĪļÖŹµÄĮ攣
£Ø6£©ÄÜĖµĆ÷øĆ·“Ó¦ŅŃ¾“ļµ½Ę½ŗāדĢ¬µÄŹĒ£Ø £©
a£® v£ØSO3£©£½2v£ØO2£© b£®ČŻĘ÷ÄŚŃ¹Ēæ±£³Ö²»±ä
c£®vÄę£ØSO2£©=2vÕż£ØO2£© d£®ŗćČŻČŻĘ÷ÄŚĪļÖŹµÄĆܶȱ£³Ö²»±ä
£Ø7£©ĪŖŹ¹øĆ·“Ó¦µÄ·“Ó¦ĖŁĀŹŌö“ó£¬ĒŅĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶ÆµÄŹĒ£Ø £©
a£®¼°Ź±·ÖĄė³öSO3ĘųĢå b£®ŹŹµ±ÉżøßĪĀ¶Č
c£®Ōö“óO2µÄÅØ¶Č d£®Ń”Ōńøߊ§µÄ“߻ƼĮ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com