
µŖŹĒµŲĒņÉĻŗ¬Įæ·įø»µÄŅ»ÖÖŌŖĖŲ£¬Ę䵄֏¼°»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²ś”¢Éś»īÖŠÓŠ×ÅÖŲŅŖ×÷ÓĆ”£
£Ø1£©Ņ»¶ØĪĀ¶ČĻĀ£¬ŌŚ1LČŻ»żŗć¶ØµÄĆܱÕČŻĘ÷ÖŠ³äČė2 mol N2ŗĶ8molH2²¢·¢Éś·“Ó¦”£10min“ļĘ½ŗā£¬²āµĆ°±ĘųµÄÅضČĪŖ0£®4 mol”¤L£1£¬“ĖŹ±µŖĘųµÄ×Ŗ»ÆĀŹĪŖ________”£ČōĻėĢįøß°±ĘųµÄ²śĀŹ£¬øł¾Ż»ÆŃ§Ę½ŗāŅʶÆŌĄķ£¬Ģį³öŗĻĄķµÄ½ØŅé______________£ØŠ“³öŅ»Ģõ¼“æÉ£©”£
£Ø2£©ČēĶ¼ŹĒ1mol NO2£Øg£©ŗĶ1mol CO£Øg£©·“Ӧɜ³Élmol CO2£Øg£©ŗĶ1 mol NO£Øg£©¹ż³ĢÖŠÄÜĮæ±ä»ÆŹ¾ŅāĶ¼£¬ĒėŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½_____________________”£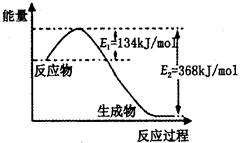
£Ø3£©ŌŚČŻ»żŗć¶ØµÄĆܱÕČŻĘ÷ÖŠ£¬½ųŠŠČēĻĀ·“Ó¦£ŗN2£Øg£©£«3H2£Øg£©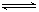 2NH3£Øg£©”÷H£¼0£¬ĘäĘ½ŗā³£ŹżKÓėĪĀ¶ČTµÄ¹ŲĻµČēĻĀ±ķ£ŗ
2NH3£Øg£©”÷H£¼0£¬ĘäĘ½ŗā³£ŹżKÓėĪĀ¶ČTµÄ¹ŲĻµČēĻĀ±ķ£ŗ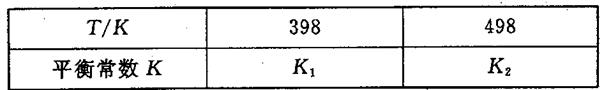
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½£ŗK£½_____________£»
¢ŚŹŌÅŠ¶ĻK1__________K2£ØĢīŠ“”°£¾”±”°£½”±»ņ”°£¼”±£©£»
¢ŪNH3£Øg£©Č¼Éյķ½³ĢŹ½ĪŖ£ŗ4NH3£Øg£©£«7O2£Øg£©£½4NO2£Øg£©£«6H2O£Øl£©£¬ŅŃÖŖ£ŗ
¢Ł2H2£Øg£©£«O2£Øg£©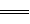 2H2O£Øl£© ”÷H£½£483£®6 kJ£Æmol
2H2O£Øl£© ”÷H£½£483£®6 kJ£Æmol
¢ŚN2£Øg£©£«2O2£Øg£© 2NO2£Øg£© ”÷H£½£«67£®8 kJ£Æmol
2NO2£Øg£© ”÷H£½£«67£®8 kJ£Æmol
¢ŪN2£Øg£©£«3H2£Øg£© 2NH3£Øg£© ”÷H£½£92£®0 kJ£Æmol
2NH3£Øg£© ”÷H£½£92£®0 kJ£Æmol
Ēė¼ĘĖćNH3£Øg£©µÄČ¼ÉÕČČ________kJ£Æmol”£
£Ø1£©10%£»Ōö“ó·“Ó¦ĪļµÄÅØ¶Č”¢½µĪĀ”¢Ōö“óŃ¹ĒæµČ£»
£Ø2£©NO2£Øg£©+CO£Øg£©=CO2£Øg£©+NO£Øg£©”÷H=-234 kJ£Æmol£»
£Ø3£©¢ŁK=c2(NH3)/ c(N2) c3(H2)£»¢Ś£¾£»¢Ū282.8
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©øł¾ŻĢāŅāÖŖ£¬Ę½ŗāŹ±°±ĘųµÄĪļÖŹµÄĮæĪŖ0.4mol£¬øł¾ŻN2£Øg£©£«3H2£Øg£© 2NH3£Øg£©ÖŖ£¬×Ŗ»ÆµÄµŖĘųµÄĪļÖŹµÄĮæĪŖ0.2mol£¬µŖĘųµÄ×Ŗ»ÆĀŹĪŖ10%£»ČōĻėĢįøß°±ĘųµÄ²śĀŹ£¬øł¾Ż»ÆŃ§Ę½ŗāŅʶÆŌĄķ£¬Ģį³öŗĻĄķµÄ½ØŅé£ŗŌö“ó·“Ó¦ĪļµÄÅØ¶Č”¢½µĪĀ”¢Ōö“óŃ¹ĒæµČ£»£Ø2£©ÓÉĢāøųĶ¼ĻńÖŖ£¬øĆ·“Ó¦µÄģŹ±ä”÷H=E1”ŖE2=134KJ/mol”Ŗ368KJ/mol=”Ŗ234KJ/mol£¬ČČ»Æѧ·½³ĢŹ½ĪŖNO2£Øg£©+CO£Øg£©=CO2£Øg£©+NO£Øg£©”÷H=”Ŗ234 kJ£Æmol£¬£Ø3£©¢Łøł¾Ż»ÆŃ§Ę½ŗā³£ŹżµÄ¶ØŅ劓³ö£¬K=c2(NH3)/ c(N2) c3(H2)£»¢ŚŗĻ³É°±µÄ·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ÉżøßĪĀ¶Č£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬Ę½ŗā³£Źż¼õŠ”£¬ĖłŅŌK1£¾K2£»¢Ūøł¾ŻøĒĖ¹¶ØĀÉ£ŗ¢Ł”Į3+¢Ś”Į2”Ŗ¢Ū”Į2µĆ£¬4NH3£Øg£©£«7O2£Øg£©£½4NO2£Øg£©£«6H2O£Øl£©£¬”÷H=”Ŗ1131.2 kJ£Æmol£¬½įŗĻČ¼ÉÕČȵĶØŅåÖŖ£¬NH3£Øg£©µÄČ¼ÉÕČČ282.8kJ£Æmol”£
2NH3£Øg£©ÖŖ£¬×Ŗ»ÆµÄµŖĘųµÄĪļÖŹµÄĮæĪŖ0.2mol£¬µŖĘųµÄ×Ŗ»ÆĀŹĪŖ10%£»ČōĻėĢįøß°±ĘųµÄ²śĀŹ£¬øł¾Ż»ÆŃ§Ę½ŗāŅʶÆŌĄķ£¬Ģį³öŗĻĄķµÄ½ØŅé£ŗŌö“ó·“Ó¦ĪļµÄÅØ¶Č”¢½µĪĀ”¢Ōö“óŃ¹ĒæµČ£»£Ø2£©ÓÉĢāøųĶ¼ĻńÖŖ£¬øĆ·“Ó¦µÄģŹ±ä”÷H=E1”ŖE2=134KJ/mol”Ŗ368KJ/mol=”Ŗ234KJ/mol£¬ČČ»Æѧ·½³ĢŹ½ĪŖNO2£Øg£©+CO£Øg£©=CO2£Øg£©+NO£Øg£©”÷H=”Ŗ234 kJ£Æmol£¬£Ø3£©¢Łøł¾Ż»ÆŃ§Ę½ŗā³£ŹżµÄ¶ØŅ劓³ö£¬K=c2(NH3)/ c(N2) c3(H2)£»¢ŚŗĻ³É°±µÄ·“Ó¦ĪŖ·ÅČČ·“Ó¦£¬ÉżøßĪĀ¶Č£¬Ę½ŗāÄęĻņŅĘ¶Æ£¬Ę½ŗā³£Źż¼õŠ”£¬ĖłŅŌK1£¾K2£»¢Ūøł¾ŻøĒĖ¹¶ØĀÉ£ŗ¢Ł”Į3+¢Ś”Į2”Ŗ¢Ū”Į2µĆ£¬4NH3£Øg£©£«7O2£Øg£©£½4NO2£Øg£©£«6H2O£Øl£©£¬”÷H=”Ŗ1131.2 kJ£Æmol£¬½įŗĻČ¼ÉÕČȵĶØŅåÖŖ£¬NH3£Øg£©µÄČ¼ÉÕČČ282.8kJ£Æmol”£
æ¼µć£ŗæ¼²é»ÆŃ§Ę½ŗā¼ĘĖć”¢Ę½ŗāŅʶÆŌĄķ”¢»ÆŃ§Ę½ŗā³£Źż¼°Ó°ĻģŅņĖŲ”¢ČČ»Æѧ·½³ĢŹ½µÄŹéŠ“¼°Č¼ÉÕČȵÄøÅÄī”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(1)¼×“¼ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤²śĘ·£¬æÉĄūÓĆ¼×“¼“ß»ÆĶŃĒāÖʱø¼×Č©”£¼×Č©ÓėĘųĢ¬¼×“¼×Ŗ»ÆµÄÄÜĮæ¹ŲĻµČēĶ¼ĖłŹ¾”£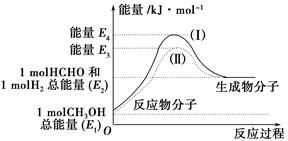
·“Ó¦¹ż³ĢÖŠµÄÄÜĮæ¹ŲĻµ
¢Ł¼×“¼“ß»ÆĶŃĒā×Ŗ»ÆĪŖ¼×Č©µÄ·“Ó¦ŹĒ________(Ģī”°ĪüČČ”±»ņ”°·ÅČČ”±)·“Ó¦”£
¢Ś¹ż³Ģ¢ńÓė¹ż³Ģ¢ņµÄ·“Ó¦ČČŹĒ·ńĻąĶ¬£æ____________ŌŅņŹĒ__________________________________”£
¢ŪŠ“³ö¼×“¼“ß»ÆĶŃĒā×Ŗ»ÆĪŖ¼×Č©µÄČČ»Æѧ·“Ó¦·½³ĢŹ½________________________________”£
(2)ŅŃÖŖ£ŗ¢ŁCH3OH(g)£«H2O(g)=CO2(g)£«3H2(g)””¦¤H£½£«49.0 kJ”¤mol£1
¢ŚCH3OH(g)£«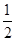 O2(g)=CO2(g)£«2H2(g)””¦¤H£½£192.9 kJ”¤mol£1
O2(g)=CO2(g)£«2H2(g)””¦¤H£½£192.9 kJ”¤mol£1
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£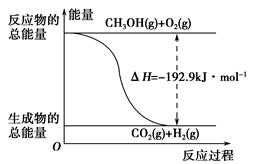
| A£®CH3OH×Ŗ±ä³ÉH2µÄ¹ż³ĢŅ»¶ØŅŖĪüŹÕÄÜĮæ |
| B£®¢Ł·“Ó¦ÖŠ£¬·“Ó¦ĪļµÄ×ÜÄÜĮæøßÓŚÉś³ÉĪļµÄ×ÜÄÜĮæ |
C£®øł¾Ż¢ŚĶĘÖŖ·“Ó¦£ŗCH3OH(l)£«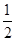 O2(g)=CO2(g)£«2H2(g)µÄ¦¤H£¾£192.9 kJ”¤mol£1 O2(g)=CO2(g)£«2H2(g)µÄ¦¤H£¾£192.9 kJ”¤mol£1 |
| D£®·“Ó¦¢ŚµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÄÜŌ“µÄæŖ·¢”¢ĄūÓĆÓėČĖĄąÉē»įµÄæɳ֊ų·¢Õ¹Ļ¢Ļ¢Ļą¹Ų£¬³ä·ÖĄūÓĆŗĆÄÜŌ“ŹĒ°ŚŌŚČĖĄąĆęĒ°µÄÖŲ“óæĪĢā”£
¢ń£®ŅŃÖŖ£ŗ¢ŁFe2O3(s)£«3C(ŹÆÄ«)£½2Fe(s)£«3CO(g) ¦¤H£½a kJ”¤mol£1
¢ŚCO(g)£«l/2O2(g)£½CO2(g) ¦¤H£½b kJ”¤mol£1
¢ŪC(ŹÆÄ«)£«O2(g)£½CO2(g) ¦¤H£½c kJ”¤mol£1
Ōņ·“Ó¦4Fe(s)£«3O2(g)£½2Fe2O3(s)µÄģŹ±ä¦¤H£½ kJ”¤mol£1”£
¢ņ£®ŅĄ¾ŻŌµē³ŲµÄ¹¹³ÉŌĄķ£¬ĻĀĮŠ»Æѧ·“Ó¦ŌŚĄķĀŪÉĻæÉŅŌÉč¼Ę³ÉŌµē³ŲµÄŹĒ £ØĢīŠņŗÅ)”£
A£®C(s)£«CO2(g)£½2CO(g) ¦¤H£¾0 B£®NaOH(aq)£«HCl(aq)£½NaCl(aq)£«H2O(l) ¦¤H£¼0
C£®2H2O(l)£½2H2(g)£«O2(g) ¦¤H£¾0 D£®CH4(g)£«2O2(g)£½CO2(g)£«2H2O(l) ¦¤H£¼0
ČōŅŌĻ”ĮņĖįĪŖµē½āÖŹČÜŅŗ£¬ŌņøĆŌµē³ŲµÄÕż¼«·“Ó¦Ź½ĪŖ ”£
¢ó£®ĒāĘų×÷ĪŖŅ»ÖÖĀĢÉ«ÄÜŌ“£¬¶ŌÓŚČĖĄąµÄÉś“ęÓė·¢Õ¹¾ßÓŠŹ®·ÖÖŲŅŖµÄŅāŅ唣
£Ø1£©ŹµŃé²āµĆ£¬ŌŚĶس£ĒéæöĻĀ£¬1 g H2ĶźČ«Č¼ÉÕÉś³ÉŅŗĢ¬Ė®£¬·Å³ö142.9 kJČČĮ攣ŌņH2Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓĆĒāĘųŗĻ³É°±µÄČČ»Æѧ·½³ĢŹ½ĪŖN2(g)£«3H2(g)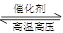 2NH3(g) ¦¤H£½£92.4 kJ”¤mol£1
2NH3(g) ¦¤H£½£92.4 kJ”¤mol£1
¢ŁŅ»¶ØĢõ¼žĻĀ£¬ĻĀĮŠŠšŹöæÉŅŌĖµĆ÷øĆ·“Ó¦ŅŃ“ļĘ½ŗāדĢ¬µÄŹĒ ”£
A£®¦ŌÕż(N2)£½¦ŌÄę(NH3)
B£®ø÷ĪļÖŹµÄĪļÖŹµÄĮæĻąµČ
C£®»ģŗĻĘųĢåµÄĪļÖŹµÄĮæ²»ŌŁ±ä»Æ
D£®»ģŗĻĘųĢåµÄĆÜ¶Č²»ŌŁ±ä»Æ
¢ŚĻĀĶ¼±ķŹ¾ŗĻ³É°±·“Ó¦“ļµ½Ę½ŗāŗó£¬Ćæ“ĪÖ»øıäĪĀ¶Č”¢Ń¹Ē攢“߻ƼĮÖŠµÄijŅ»Ģõ¼ž£¬·“Ó¦ĖŁĀŹ¦ŌÓėŹ±¼ätµÄ¹ŲĻµ”£ĘäÖŠ±ķŹ¾Ę½ŗā»ģŗĻĪļÖŠµÄNH3µÄŗ¬Įæ×īøßµÄŅ»¶ĪŹ±¼äŹĒ ”£Ķ¼ÖŠt3Ź±øıäµÄĢõ¼žæÉÄÜŹĒ ”£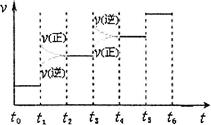
¢ŪĪĀ¶ČĪŖT”ꏱ£¬½«4a mol H2ŗĶ2a mol N2·ÅČė0.5 LĆܱÕČŻĘ÷ÖŠ£¬³ä·Ö·“Ó¦ŗó²āµĆN2µÄ×Ŗ»ÆĀŹĪŖ50%£¬Ōņ·“Ó¦µÄĘ½ŗā³£ŹżĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©ŅŃÖŖ·“Ó¦¼°¼øÖÖĪļÖŹÖŠ»Æѧ¼ü¶ĻĮŃŹ±µÄÄÜĮæ±ä»ÆČēĻĀĖłŹ¾£ŗ
H2(g)£«Cl2(g)£½2HCl(g) ”÷H£½£184kJ/mol
4HCl (g)£«O2(g)£½2Cl2 (g) £«2H2O (g) ”÷H£½£115.6kJ/mol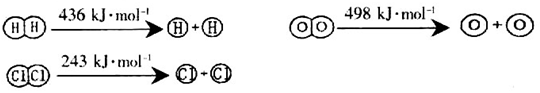
¢ŁH2ÓėO2·“Ӧɜ³ÉĘųĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½ĪŖ_________________________________£»
¢Ś¶ĻæŖ1mol H”ŖO¼üĖłŠčÄÜĮæŌ¼ĪŖ_________________________kJ”£
£Ø2£©ŅŃÖŖij·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ£ŗK£½ ,ĖüĖł¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
,ĖüĖł¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
£Ø3£©ŅŃÖŖ·“Ó¦N2(g)£«3H2(g) 2NH3(g) ”÷H£¼0ŌŚ400”ꏱK£½0.5£¬“ĖĢõ¼žĻĀŌŚ0.5LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠøĆ·“Ó¦£¬Ņ»¶ĪŹ±¼äŗ󣬲āµĆN2”¢H2”¢NH3µÄĪļÖŹµÄĮæ·Ö±šĪŖ2mol”¢1mol”¢2mol£¬Ōņ“ĖŹ±·“Ó¦¦Ō(N2)Õż______¦Ō(N2)Äę£ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©”£ÓūŹ¹µĆøĆ·“Ó¦µÄ»Æѧ·“Ó¦ĖŁĀŹ¼Óæģ£¬Ķ¬Ź±Ź¹Ę½ŗāŹ±NH3µÄĢå»ż°Ł·ÖŹżŌö¼Ó£¬æɲÉČ”µÄ“ėŹ©ŹĒ_______£ØĢīŠņŗÅ£©”£
2NH3(g) ”÷H£¼0ŌŚ400”ꏱK£½0.5£¬“ĖĢõ¼žĻĀŌŚ0.5LµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠøĆ·“Ó¦£¬Ņ»¶ĪŹ±¼äŗ󣬲āµĆN2”¢H2”¢NH3µÄĪļÖŹµÄĮæ·Ö±šĪŖ2mol”¢1mol”¢2mol£¬Ōņ“ĖŹ±·“Ó¦¦Ō(N2)Õż______¦Ō(N2)Äę£ØĢī”°£¾”±”¢”°£¼”±»ņ”°£½”±£©”£ÓūŹ¹µĆøĆ·“Ó¦µÄ»Æѧ·“Ó¦ĖŁĀŹ¼Óæģ£¬Ķ¬Ź±Ź¹Ę½ŗāŹ±NH3µÄĢå»ż°Ł·ÖŹżŌö¼Ó£¬æɲÉČ”µÄ“ėŹ©ŹĒ_______£ØĢīŠņŗÅ£©”£
A.ĖõŠ”Ģå»żŌö“óŃ¹Ēæ B.ÉżøßĪĀ¶Č
C.¼Ó“߻ƼĮ D.Ź¹°±ĘųŅŗ»ÆŅĘ×ß
£Ø4£©ŌŚŅ»¶ØĢå»żµÄĆܱÕČŻĘ÷ÖŠ½ųŠŠČēĻĀ»Æѧ·“Ó¦£ŗA(g)£«3B(g) 2C(g)£«D(s) ”÷H£¬Ęä»ÆŃ§Ę½ŗā³£ŹżKÓėTµÄ¹ŲĻµČēĻĀ±ķ£ŗ
2C(g)£«D(s) ”÷H£¬Ęä»ÆŃ§Ę½ŗā³£ŹżKÓėTµÄ¹ŲĻµČēĻĀ±ķ£ŗ
| T/K | 300 | 400 | 500 | ”” |
| K/(mol”¤L£1)2 | 4”Į106 | 8”Į107 | 1.2”Į109 | ”” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø¢ń£©¼×“¼Č¼ĮĻµē³Ų£ØDNFC£©±»ČĻĪŖŹĒ21ŹĄ¼Ķµē¶ÆĘū³µ×ī¼ŃŗņŃ”¶ÆĮ¦Ō“”£
£Ø1£©25”ę”¢101 kPaŹ±£¬1 mol CH3OHĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄŃõ»ÆĪļ·Å³öČČĮæ726.51 kJ/mol£¬Ōņ¼×“¼Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ:”””””””” ”” ”£
£Ø2£©¼×“¼Č¼ĮĻµē³ŲµÄ½į¹¹Ź¾ŅāĶ¼ČēĻĀ”£¼×“¼½ųČė ¼«£ØĢī”°Õż”±»ņ”°øŗ”±£©£¬Š“³öøĆ¼«µÄµē¼«·“Ó¦Ź½ ”£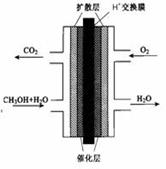
£Ø¢ņ£©Ē¦Šīµē³ŲŹĒµäŠĶµÄæɳäŠĶµē³Ų£¬ĖüµÄÕżøŗ¼«øō°åŹĒ¶čŠŌ²ÄĮĻ£¬µē³Ų×Ü·“Ó¦Ź½ĪŖ£ŗPb£«PbO2£«4H£«£«2SO42£ 2PbSO4£«2H2O£¬Ēė»Ų“šĻĀĮŠĪŹĢā£Ø²»æ¼ĀĒĒā”¢ŃõµÄŃõ»Æ»¹Ō£©£ŗ
2PbSO4£«2H2O£¬Ēė»Ų“šĻĀĮŠĪŹĢā£Ø²»æ¼ĀĒĒā”¢ŃõµÄŃõ»Æ»¹Ō£©£ŗ
£Ø1£©·ÅµēŹ±£ŗÕż¼«µÄµē¼«·“Ó¦Ź½ŹĒ µē½āŅŗÖŠH2SO4µÄÅØ¶Č½«±ä”””””” £»
£Ø2£©ŌŚĶźČ«·Åµēŗľ”PbO2ŗĶPbŹ±£¬Čō°“ÓŅĶ¼Į¬½Ó£¬µē½āŅ»¶ĪŹ±¼äŗó£¬ŌņŌŚAµē¼«ÉĻÉś³É””””””””Bµē¼«ÉĻÉś³É”””””” ”””£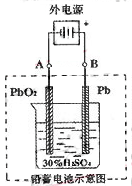
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µŖæÉŠĪ³É¶ąÖÖŃõ»ÆĪļ£¬ČēNO”¢NO2”¢N2O4µČ”£ŅŃÖŖNO2ŗĶN2O4µÄ½į¹¹Ź½·Ö±šŹĒ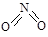 ŗĶ
ŗĶ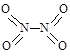 ”£ŹµŃé²āµĆN£N¼ü¼üÄÜĪŖ167kJ”¤mol£1£¬ NO2ÖŠµŖŃõ¼üµÄĘ½¾ł¼üÄÜĪŖ466 kJ”¤mol£1£¬N2O4ÖŠµŖŃõ¼üµÄĘ½¾ł¼üÄÜĪŖ438.5 kJ”¤mol£1”£
”£ŹµŃé²āµĆN£N¼ü¼üÄÜĪŖ167kJ”¤mol£1£¬ NO2ÖŠµŖŃõ¼üµÄĘ½¾ł¼üÄÜĪŖ466 kJ”¤mol£1£¬N2O4ÖŠµŖŃõ¼üµÄĘ½¾ł¼üÄÜĪŖ438.5 kJ”¤mol£1”£
£Ø1£©Š“³öN2O4×Ŗ»ÆĪŖNO2µÄČČ»Æѧ·½³ĢŹ½£ŗ
£Ø2£©¶Ō·“Ó¦N2O4(g) 2NO2(g)£¬ŌŚĪĀ¶ČĪŖT1”¢T2Ź±£¬Ę½ŗāĢåĻµÖŠNO2µÄĢå»ż·ÖŹżĖęŃ¹Ēæ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
2NO2(g)£¬ŌŚĪĀ¶ČĪŖT1”¢T2Ź±£¬Ę½ŗāĢåĻµÖŠNO2µÄĢå»ż·ÖŹżĖęŃ¹Ēæ±ä»ÆĒśĻßČēĶ¼ĖłŹ¾”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ 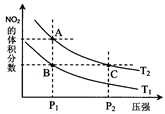
A£®A”¢CĮ½µćµÄ·“Ó¦ĖŁĀŹ£ŗA£¾C
B£®B”¢CĮ½µćµÄĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ£ŗB£¼C
C£®A”¢CĮ½µćĘųĢåµÄŃÕÉ«£ŗAÉī£¬CĒ³
D£®ÓÉדĢ¬Bµ½×“Ģ¬A£¬æÉŅŌÓĆ¼ÓČȵķ½·Ø
£Ø3£©ŌŚ100”ꏱ£¬½«0.40molµÄNO2ĘųĢå³äČė2 L³éæÕµÄĆܱÕČŻĘ÷ÖŠ£¬ĆæøōŅ»¶ØŹ±¼ä¾Ķ¶ŌøĆČŻĘ÷ÄŚµÄĪļÖŹ½ųŠŠ·ÖĪö£¬µĆµ½ČēĻĀ±ķŹż¾Ż£ŗ
| Ź±¼ä£Øs£© | 0 | 20 | 40 | 60 | 80 |
| n(NO2)/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n(N2O4)/mol | 0.00 | 0.050 | n2 | 0.080 | 0.080 |
 N2O4µÄĘ½ŗā³£ŹżK½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£
N2O4µÄĘ½ŗā³£ŹżK½« £ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅĄ¾ŻŹĀŹµ£¬Š“³öĻĀĮŠ·“Ó¦µÄČČ»Æѧ·“Ó¦·½³ĢŹ½”£
£Ø1£©ŌŚ25”ę”¢101kPaĻĀ£¬1g¼×“¼ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®Ź±·ÅČČ22£®68kJ”£Ōņ±ķŹ¾¼×“¼Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ČōŹŹĮæµÄN2ŗĶO2ĶźČ«·“Ó¦£¬ĆæÉś³É23gNO2ŠčŅŖĪüŹÕ16£®95kJČČĮ棬Ōņ±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ŌŚC2H2£ØĘųĢ¬£©ĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®µÄ·“Ó¦ÖŠ£¬ĆæÓŠ5NAøöµē×Ó×ŖŅĘŹ±£¬·Å³ö650kJµÄČČĮ棬Ōņ±ķŹ¾øĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ _________________________________________________”£
£Ø4£©ŅŃÖŖ²šæŖ1molH”ŖH¼ü£¬1molN”ŖH¼ü£¬1molN”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢391kJ”¢946kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©8gŅŗĢ¬µÄCH3OHŌŚŃõĘųÖŠĶźČ«Č¼ÉÕ£¬Éś³É¶žŃõ»ÆĢ¼ĘųĢåŗĶŅŗĢ¬Ė®Ź±ŹĶ·Å³öQ kJµÄČČĮ攣ŹŌŠ“³öŅŗĢ¬CH3OHČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŌŚ»Æѧ·“Ó¦¹ż³ĢÖŠ£¬ĘĘ»µ¾É»Æѧ¼üŠčŅŖĪüŹÕÄÜĮ棬ŠĪ³ÉŠĀ»Æѧ¼üÓÖ»įŹĶ·ÅÄÜĮ攣
| »Æѧ¼ü | H”ŖH | N”ŖH | N”ŌN |
| ¼üÄÜ/kJ”¤mol£1 | 436 | 391 | 945 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¢ń”¢ĻĀĮŠŹµŃé²Ł×÷»ņ¶ŌŹµŃéŹĀŹµµÄĆčŹöÕżČ·µÄŹĒ____________________
¢Ł ÓĆĮæĶ²ĮæČ”Ļ”ĮņĖįČÜŅŗ8.0mL£»
¢ŚÖŠŗĶČČµÄ²ā¶ØŹµŃéÖŠ£¬æÉÓĆ½šŹōĖæ£Ø°ō£©“śĢę»·ŠĪ½Į°č²£Į§°ō£»
¢ŪÓĆČȵÄÅØŃĪĖįĻ“µÓø½×ÅÓŠMnO2µÄŹŌ¹Ü£»
¢ÜŌŚĮņĖįĶ¾§Ģå½į¾§Ė®ŗ¬ĮæµÄ²ā¶ØÖŠ£¬Čō¼ÓČČŗóµÄĪŽĖ®ĮņĖįĶ·ŪÄ©±ķĆę·¢ŗŚ£¬ŌņĖł²ā½į¾§Ė®ŗ¬ĮææÉÄÜ»įĘ«øß £»
¢ŻFe(OH)3½ŗĢåÓėFeCl3ČÜŅŗæÉÓĆ¹żĀĖµÄ·½·Ø·ÖĄė£»
¢ŽÓĆ¼īŹ½µĪ¶Ø¹ÜĮæČ”KMnO4ČÜŅŗ20.50mL £»
¢ß½«Ė®ŃŲÉÕ±ÄŚ±Ś»ŗ»ŗ×¢ČėÅØĮņĖįÖŠ£¬²»¶ĻÓĆ²£Į§°ō½Į°čŅŌĻ”ŹĶÅØĮņĖį£»
¢ąÓĆŹŖČóµÄpHŹŌÖ½²āĮæijČÜŅŗpHŹ±£¬²āĮæÖµŅ»¶Ø±ČÕꏵֵŠ”£»
¢įŠæŗĶŅ»¶ØĮæĻ”ĮņĖį·“Ó¦£¬ĪŖ¼ÓæģĖŁĀŹ¶ų²»Ó°ĻģH2µÄĮææÉĻņČÜŅŗÖŠ¼ÓŹŹĮæCu(NO3)2¾§Ģ唣
¢ņ”¢2013Äź³õ£¬Īķö²ĢģĘų¶ą“ĪĖĮÅ°Ģģ½ņ”¢±±¾©µČµŲĒų”£ĘäÖŠ£¬Č¼ĆŗŗĶĘū³µĪ²ĘųŹĒŌģ³ÉæÕĘųĪŪČ¾µÄŌŅņÖ®Ņ»”£
£Ø1£©Ęū³µĪ²Ęų¾»»ÆµÄÖ÷ŅŖŌĄķĪŖ£ŗ2NO(g) + 2CO(g) 2CO2(g)+ N2(g)”£”÷H£¼0
2CO2(g)+ N2(g)”£”÷H£¼0
¢ŁøĆ·“Ó¦Ę½ŗā³£Źż±ķ“ļŹ½
¢ŚČōøĆ·“Ó¦ŌŚ¾ųČČ”¢ŗćČŻµÄĆܱÕĢåĻµÖŠ½ųŠŠ£¬ĻĀĮŠŹ¾ŅāĶ¼ÕżČ·ĒŅÄÜĖµĆ÷·“Ó¦ŌŚ½ųŠŠµ½t1Ź±æĢ“ļµ½Ę½ŗāדĢ¬µÄŹĒ £ØĢī“śŗÅ£©”£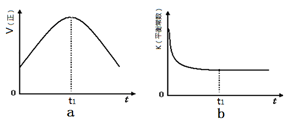
£Ø2£©Ö±½ÓÅÅ·ÅĆŗČ¼ÉÕ²śÉśµÄŃĢĘų»įŅżĘšŃĻÖŲµÄ»·¾³ĪŹĢā”£
ĆŗČ¼ÉÕ²śÉśµÄŃĢĘųŗ¬µŖµÄŃõ»ÆĪļ£¬ÓĆCH4“߻ƻ¹ŌNOXæÉŅŌĻū³żµŖŃõ»ÆĪļµÄĪŪČ¾”£
ŅŃÖŖ£ŗ¢ŁCH4(g)+2NO2(g)£½N2(g)£«CO2(g)+2H2O(g)”””÷H£½£867 kJ/mol
¢Ś2NO2(g)  N2O4(g) ”÷H£½£56.9 kJ/mol
N2O4(g) ”÷H£½£56.9 kJ/mol
¢ŪH2O(g) £½ H2O(l) ¦¤H £½ £44.0 kJ£Æmol
Š“³öCH4“߻ƻ¹ŌN2O4(g)Éś³ÉN2ŗĶH2O(l)µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com