
a��b��c��d
Ϊԭ��������������Ķ�����Ԫ�أ�b��c��dͬ���ڣ���a��b��c��d����Ԫ���γɵij�����ʽ��A����ͼ��ʾ��ת����ϵ(ͼ��ÿ����ĸ��ʾһ�ֵ��ʻ���)��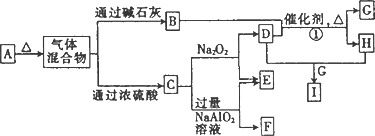
(1)C��ԭ�ӽṹʾ��ͼΪ________��
(2)д���������ʵĻ�ѧʽ��F________��I________��
(3)д����Ӧ�ٵĻ�ѧ����ʽ��________��
(4)
��A��E��ɵĹ�������X g����ˮ�����Һ����������������I��ϡ��Һ����ü���I��Һ�����������C�����(��״��)���±���ʾ��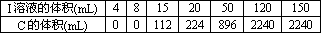
��I��Һ�����ʵ���Ũ��Ϊ________��
��x
��ֵΪ________��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| c2(SO3) |
| c2(SO2)?c(O2) |
| c2(SO3) |
| c2(SO2)?c(O2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | A | B | C | D |
| ���ʻ�ṹ��Ϣ | ��ҵ��ͨ������Һ̬��������䵥�ʣ���������ȼ | ��̬�⻯���Լ��� | +3�������ӵĺ�������Ų�����ԭ����ͬ | ��������ԭ�Ӱ뾶��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com