
(8��)�⻯����ʵ�����г��õķ����Լ�����ҵ������м��ԭ���Ʊ�NaI����������ͼ��
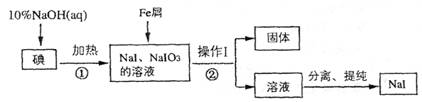
��ش��������⣺
(1)
�жϷ�Ӧ���е��Ƿ�Ӧ��ȫ�ķ�����_____________ ________
________
(2) ����I��������_____________________��
(3) ��Ӧ�ٵ����ӷ���ʽΪ_____________________
(4) ��Ӧ����NaIO3��Fe���ʻ�ԭΪNaI��ͬʱ����Fe(OH)3,�÷�Ӧ�Ļ�ѧ����ʽ��______��
�ڸ÷�Ӧ������99 g NaIO3����ԭ����ת�Ƶ��ӵ����ʵ���Ϊ_______mol
��8�֣���1��ȡ������Ӧ�����Һ���Թ��У����뼸�ε�����Һ������Һδ��������֤�����ѷ�Ӧ��ȫ����֮����δ��Ӧ��ȫ����1�֣�����ȡ������Ӧ�����Һ���Թ��У����뼸��CCl4�������ã����²�Һ�����ɫ��֤�����ѷ�Ӧ��ȫ�����²�Һ����Ϻ�ɫ��֤����δ��Ӧ��ȫ����
��2�����ˣ�1�֣� ��3��3I2 + 6OH��=5I��+ IO3�� + 3H2O ��2�֣�
��4��2Fe+ NaIO3+ 3H2O=2Fe(OH)3��+NaI ��2�֣� 3��2�֣�
�����������⿼�鹤ҵ�����й�֪ʶ����1�����ʵ������۱�������˿��õ�����Һ���顣Ҳ�ɲ�����ȡ�ķ��������ⵥ����ȡ����������ȡ���е���ɫ����ȡ������Ӧ�����Һ���Թ��У����뼸�ε�����Һ������Һδ��������֤�����ѷ�Ӧ��ȫ����֮����δ��Ӧ��ȫ����ȡ������Ӧ�����Һ���Թ��У����뼸��CCl4�������ã����²�Һ�����ɫ��֤�����ѷ�Ӧ��ȫ�����²�Һ����Ϻ�ɫ��֤����δ��Ӧ��ȫ����(2)������ͼ��������I�����ƺ���õ����ǹ������Һ���ʲ���I�������ǹ��ˡ�(3)�ӷ�Ӧǰ����Կ�������Ӧ��ΪNaOH��I2������ΪNaI��NaIO3���ʷ�Ӧ�����ӷ���ʽΪ3I2 + 6OH��=5I��+ IO3�� + 3H2O����4��NaIO3��Fe���ʻ�ԭΪNaI��ͬʱ����Fe(OH)3,�÷�Ӧ�Ļ�ѧ����ʽ��2Fe+ NaIO3+ 3H2O=2Fe(OH)3��+NaI���ӷ���ʽǰ�ϼ۵������������ڸ÷�Ӧ������99 g NaIO3(��0.5mol)����ԭΪNaI(�õ�6mol����)����ת�Ƶ��ӵ����ʵ���Ϊ3mol


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)�⻯����ʵ�����г��õķ����Լ�����ҵ������м��ԭ���Ʊ�NaI����������ͼ��
��ش��������⣺
(1)�жϷ�Ӧ���е��Ƿ�Ӧ��ȫ�ķ�����_____________________
(2) ����I��������_____________________��
(3) ��Ӧ�ٵ����ӷ���ʽΪ_____________________
(4) ��Ӧ����NaIO3��Fe���ʻ�ԭΪNaI��ͬʱ����Fe(OH)3,�÷�Ӧ�Ļ�ѧ����ʽ��______��
�ڸ÷�Ӧ������99 gNaIO3����ԭ����ת�Ƶ��ӵ����ʵ���Ϊ_______mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
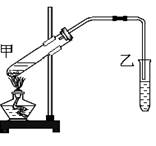
(8��) ��ͼ��ʵ�����Ʊ�����������ʵ��װ�á��Իش�
��1���Թܼ��м�����Լ�˳���ǣ� ��
A���Ҵ���Ũ���ᡢ����
B��Ũ���ᡢ�Ҵ�������
C��Ũ���ᡢ���ᡢ�Ҵ�
��2���״����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ��
��3��С�Թ����б���Na2CO3��������( )
A�������Ҵ�
B�������
C��������Һ�ܶȣ���������������������ܽ��
��4����װ���еĵ���δ������Һ��Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ������ѧ�߶���ѧ��ѧҵˮƽ�ڶ���ģ�⿼�Ի�ѧ���� ���ͣ�ʵ����
(8��) ��ͼ��ʵ�����Ʊ�����������ʵ��װ�á��Իش�
��1���Թܼ��м�����Լ�˳���ǣ� ��
A���Ҵ���Ũ���ᡢ����
B��Ũ���ᡢ�Ҵ�������
C��Ũ���ᡢ���ᡢ�Ҵ�
��2���״����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ��
��3��С�Թ����б���Na2CO3��������( )
A�������Ҵ�
B�������
C��������Һ�ܶȣ���������������������ܽ��
��4����װ���еĵ���δ������Һ��Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�߶���ѧ��ѧҵˮƽ�ڶ���ģ�⿼�Ի�ѧ���� ���ͣ�ʵ����
(8��) ��ͼ��ʵ�����Ʊ�����������ʵ��װ�á��Իش�
��1���Թܼ��м�����Լ�˳���ǣ� ��
A���Ҵ���Ũ���ᡢ����
B��Ũ���ᡢ�Ҵ�������
C��Ũ���ᡢ���ᡢ�Ҵ�
��2���״����Թ��ڷ�����Ӧ�Ļ�ѧ����ʽΪ��
��3��С�Թ����б���Na2CO3��������( )
A�������Ҵ�
B�������
C��������Һ�ܶȣ���������������������ܽ��
��4����װ���еĵ���δ������Һ��Ŀ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com