
ij�о�С������ӷ���ʽxR2+ + yH+ + O2 = mR3+ + nH2O�ķ����о�,����˵���д������
A�����ݵ���غ�,�ó�x��y�ĺ�һ������m
B������ԭ���غ�,�ó�x��m����ֵһ�����
C�����ݵ��ӵ�ʧ�غ�,�ó�x=4�Ľ���
D������������ԭ��Ӧ��ϵ�ó���R2+�ǻ�ԭ��, O2��������, R3+����������, H2O�ǻ�ԭ����
��֪ʶ�㡿������ԭ��Ӧ�Ļ����������B2 B3
���𰸽�����A ������A�����ݵ���غ�,�ó�2x+y=3m ����A����B������Rԭ���غ�ó�x��m����ֵһ����ȣ���B��ȷ��C��1Ħ��O2�õ�4Ħ�����ӣ�xĦ��RӦ��ʧȥ4Ħ�����ӣ���x=4����C��ȷ��D��R2+���ϼ������ǻ�ԭ����O2���ϼ۽�����������, R3+����������, H2O�ǻ�ԭ�����D��ȷ��
�ʴ�ѡA
��˼·�㲦�����⿼����������ԭ��Ӧ�Ļ���������ɣ��ؼ������ⷴӦ�е��غ��ϵ��


 ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
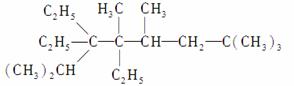
(1)д�����л���������ƻ�ṹ��ʽ��
��
________________________________________________________________________��
��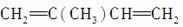 ________________________________________________________________________��
________________________________________________________________________��
��CH2===CHCOOH________________________________________________________________________��
��2,5���� 2,4����ϩ�Ľṹ��ʽ��
________________________________________________________________________
________________________________________________________________________��
(2)������ֳƻƼ���ҹ��ض�����ҩ������������е�һ���������ҹ���ѧ���о�������ṹ���£�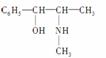
��������к��������ŵ�������________������________��(������ӡ�)��
�����и����ʣ�
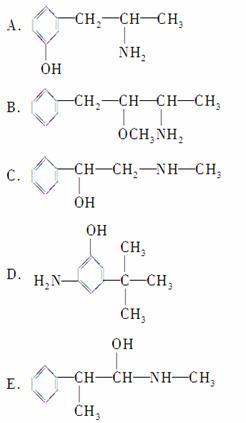
������ػ�Ϊͬ���칹�����________(����ĸ����ͬ)����Ϊͬϵ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����þ��(��Ҫ�ɷ�ΪMgCO3)����ȼ��������þ�Ĺ����������£�
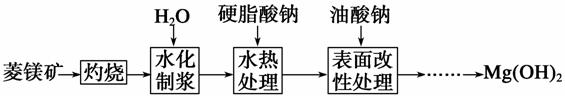
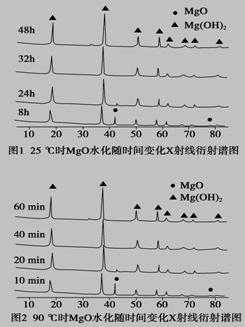
(1)��ͼ1��ͼ2���Եó��Ľ���Ϊ__________________��____________________��
(2)ˮ����ӦMgO��H2O===Mg(OH)2���Է����е�ԭ����____________________��
(3)���Ԫ�������ɺ��±���֪�����������������ȷֽ�Ĺ�����__________________��(дһ������)
��������Ԫ�صĽ������������ȷֽ��¶�/��
| LiOH | NaOH | KOH | Al(OH)3 | Mg(OH)2 | Ca(OH)2 | Ba(OH)2 |
| 924 | ���ֽ� | ���ֽ� | 140 | 258 | 390 | 700 |
(4)��֪�Ȼ�ѧ����ʽ��Mg(OH)2(s)===MgO(s)��H2O(g)����H����81.5 kJ��mol��1
��Mg(OH)2����ȼ���õ���Ҫԭ����________________________________________
________________________________________________________________________��
���볣��±ϵ(����������)���л���ϵ(����������)��ȼ����ȣ�Mg(OH)2��ȼ�����ŵ���____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)2��������Cl2����ȡ����Ӧ�����ܵõ���һ�ȴ����м��֣�
(2)��ij�����������ܶ���3.214 g/L(��״��)��������ȡ����Ӧ��õ���һ±����ֻ��һ�֣����Ƴ���ṹ��ʽ������ϵͳ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ijǿ������XO(OH)2+��Na2SO3��ԭ�������ԭ2.4��10-3mol XO(OH)2+������30mL 0.2mol/L��Na2SO3��Һ����ôXԪ�ر���ԭ��ļ�̬��
A.+2 B.+1 C.0 D.-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ʵ�����������ͽ��ͻ���۶���ȷ����
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ������Fe���м���ϡHNO3����ַ�Ӧ����KSCN��Һ | ��Һ�ʺ�ɫ | ϡHNO3��Fe����ΪFe3�� |
| B | AgI�����е���ϡKCl��Һ | �а�ɫ�������� | AgCl��AgI������ |
| C | Al������ϡHNO3�� | ������ | Al�����汻HNO3�������γ����ܵ�����Ĥ |
| D | �ò�����պȡŨ��ˮ�㵽��ɫʯ����ֽ�� | ��ֽ����ɫ | Ũ��ˮ�ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ���֮��Ϊ1��3��п��ϡ������,�����ᱻ��ԭ�IJ���ΪN2O,��Ӧ������пû��ʣ��,����˵����ȷ����(�� ��)
A.�ڴ˷�Ӧ������ֻ����ǿ������
B.��Ӧ�����Һ���ټ��������,���ٷ�����ѧ��Ӧ
C.�÷�Ӧ�б���ԭ��������δ����ԭ������֮��Ϊ1��4
D.�÷�Ӧ�б���ԭ��������δ����ԭ������֮��Ϊ1��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʾ�����ӵ�������������������ȷ����
A.��״���£�2.24L���ȼ��麬�з�����ĿΪ0.1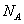
B��1 mol FeI2������������Ӧת�Ƶĵ�����Ϊ2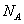
C��1 molCH3COONa ������CH3COOH����ˮ����������Һ�У�CH3COO����ĿΪ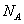
D.��״���£�22.4L����ϩ�к��еĹ��õ��Ӷ���Ϊ12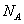
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ������˵����ȷ���ǣ� ��
A�������£�0.05 mol��L��1Fe2(SO4)3��Һ�к�Fe3+��ĿΪ0.1 NA
B����״���£�22.4L�ױ��к�C��H��ĿΪ8 NA
C�����¡���ѹ�£�1.6g O2��O3������У���ԭ�ӵ���ĿΪ0.1NA
D��0.1mol Na2O2������CO2��Ӧʱ��ת�Ƶĵ�����ĿΪ0.2 NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com