
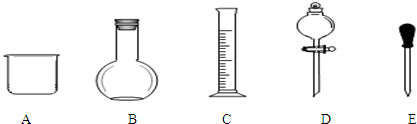
���� ��1��������Һ�����������ϸ�����������ѡȡ������
��2����������ƿ�Ĺ����ʹ��ע������������ƿ����ϡ��Ũ��Һ�����������ܽ���塢������Һ�ȣ�
��3������m=CVM������Ҫ���ʵ�����������ʱ���ӿ̶��ߣ�������Һ���ƫ������C=n/V������������
��4������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ���Һϡ��ǰ���������ʵ����ʵ������䣬�ݴ˼�����ҪŨ����������ѡ����ʵ���Ͳ��
��5�������������������ʵ����ʵ�������Һ�������Ӱ�죬����C=n/V������������
��� �⣺��1������һ�����ʵ���Ũ����Һ��һ�㲽��Ϊ�����㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ��������У�������ƽ��ҩ�ס��ձ�����������500ml����ƿ����ͷ�ιܣ��ò���������Ϊ��ƽ����ƿ����Һ©��������Ҫ������Ϊ����������500mL����ƿ��
�ʴ�Ϊ��BD����������500mL����ƿ��
��2��A������ƿ���в�������ʹ�ù�������Ҫ���µߵ�ҡ�ȣ�����ʹ��ǰ�������Ƿ�©ˮ����A��ȷ��
B������ƿΪ�������������������ܽ���塢ϡ��Ũ��Һ����B����
C������ƿΪ������������������ϡ��Ũ��Һ����C����
D�����ݺ���Ҫ�����Ƶ���Һҡ�ȣ���ȷ�IJ���Ϊ����ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת��Σ�ҡ�ȣ���D��ȷ��
�ʴ�Ϊ��BC��
��3��Ҫ����0.1mol/LNaOH��Һ480mL��Ӧѡ��500ml����ƿ����Ҫ�������Ƶ�����m=0.1mol/L��0.5L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
��4��98%���ܶ�Ϊ1.84g/cm3��Ũ��������ʵ���Ũ��C=$\frac{1000��1.84g/ml��98%}{9g/mol}$=18.4mol/L������ҪŨ��������ΪV��������Һϡ��ǰ���������ʵ����ʵ�������ã�V��18.4mol/L=0.5mol/L��500mL�����V=13.6mL��
�ʴ�Ϊ��13.6��
��5�������ձ����ܽ����ʣ�����ʱ��������������Һ��������ʧ����Һ��Ũ��ƫС��
��δ��ϴ���ձ��ڱڵ���Һת��������ƿ��������ʧ����Һ��Ũ��ƫС��
������ƿ���������ҺҺ��δ���̶��߱�ֹͣ��ˮ����Һ�����ƫС����Һ��Ũ��ƫ��
�ܽ���õ���Һ������ƿת�Ƶ�����ྻ���Լ�ƿ��ʱ����������������Һ����ã�Ũ����Ӱ�죬
�ݽ��ձ�����Һת�Ƶ�����ƿ֮ǰ������ƿ������������ˮ������Һ���������Һ��Ũ�Ȳ������Ӱ�죬
������ƿ��Һ�潫�ﵽ�̶���ʱ�����ӿ̶��ߵ�Һ�棬��Һ���ƫС��Ũ��ƫ��
�ʴ�Ϊ�������ܢݣ������ۢޣ������٢ڣ�
���� ���⿼��һ�����ʵ���Ũ����Һ�����ơ���ѧ����ʽ����ؼ��㣬ע������ƿ��ʹ�á�ע����ȷ�жϲ���ijɷ�Ϊ������Ĺؼ�����Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | dΪп�飬��Ƭ��ʴ�ӿ� | |
| B�� | dΪʯī����Ƭ���ױ���ʴ | |
| C�� | dΪп�飬��Ƭ�ϵ缫��ӦΪ��2H++2e-=H2�� | |
| D�� | dΪʯī��ʯī�ϵ缫��ӦΪ��O2+2H2O+4e-=4OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2FeC13+Cu=CuC12+2FeC12 | B�� | AlCl3+3NH3•H20=Al��0H��3��+3NH4Cl | ||
| C�� | 2Fe��0H��3$\frac{\underline{\;\;��\;\;}}{\;}$Fe203+3H20 | D�� | Na2O+CO2=Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Na2CO3��Һ����ˮ���еIJ����CaSO4��s��+CO${\;}_{3}^{2-}$��aq��=CaCO3��s��+SO${\;}_{4}^{2-}$ | |
| B�� | ��Na2S��Һ�ڿ����г��ڷ��ñ���ǣ�2S2-+O2+4H+=2S+2H2O | |
| C�� | ��NaHCO3��Һ�м�������ij���ʯ��ˮ��2HCO${\;}_{3}^{-}$+Ca2++2OH-=CaCO3��+2H2O | |
| D�� | ��Fe��NO3��3��Һ��ͨ�����SO2��2Fe3++SO2+2H2O=2Fe2++SO${\;}_{4}^{2-}$+4H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com