
| m |
| M |
| 1.1��10-10 |
| 1��10-6 |
| 0.5825g |
| 233g/mol |
| 5 |
| 2 |
| 25mL |
| 100mL |


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ���� | ���� | |
| A | ��ʢ��25mL��ˮ���ձ��еμ�5��6��FeCl3������Һ��������� | ��Һ���ɺ��ɫ���� | �Ƶ�Fe��OH��3���� |
| B | ����֧ʢ��KI3��Һ���Թ��У��ֱ�μӵ�����Һ��AgNO3��Һ�� | ǰ����Һ������ �����л�ɫ���� | KI3��Һ�д���ƽ�⣺ I3-?I2+I- |
| C | ��ij��Һ�м��������ữ���Ȼ�����Һ | �а�ɫ�������� | ����Һ��һ������SO42- |
| D | ��ˮ����ͨ�����ȵ����� | ��ĩ��� | ����ˮ�ڸ����·�����Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
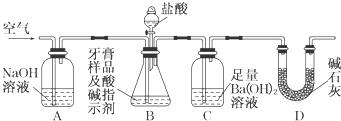
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
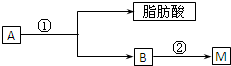
| Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
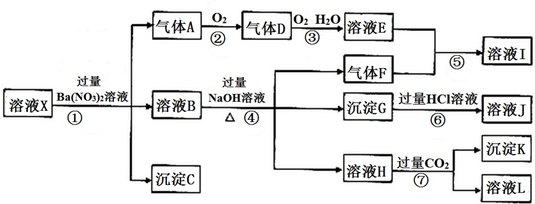
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� ���� | ||||||||
| 1 | �� | |||||||
| 2 | �� | |||||||
| 3 | �� | �� | �� | �� | ||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com