
ʯ����(17��̼ԭ�����ϵ�Һ̬���������)�ķֽ�ʵ��װ����ͼ��ʾ�����������������Թܢ��м���ʯ���ͺ�������(��ʯ���ֽ�)���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ��
ʵ�������Թܢ��м���һ��ʱ����Կ����Թ���Һ����ڣ�
�Թܢ���������Һ�����ᣬ�ŵ����͵���ζ����Һ���еμӼ��θ������������Һ��ɫ��ȥ������ʵ������ش��������⣺
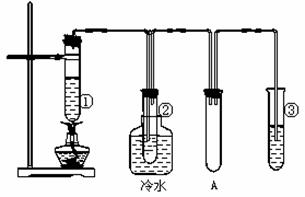
(1) װ��A��������______________________________
(2) �Թܢ��з�������Ҫ��Ӧ�У�C17H36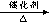 C8H18+C9H18 C8H18
C8H18+C9H18 C8H18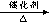 C4H10+C4H8
C4H10+C4H8
����ɽ�һ���ѽ⣬���õ�����������⣬�����Եõ��������л�����ǵĽṹ��ʽΪ_____________��________________���������л����Ϻ���һ�������¿ɾۺϳɸ߷��ӻ�����䷴Ӧ��������________��Ӧ������ܽṹΪ( )(�𰸿��ܲ�ֹһ������ͬ)
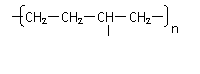
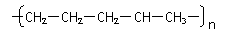 A�� B��
A�� B��
C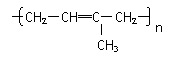
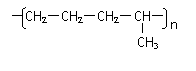
���������������������������������� D��
(3)д���Թܢ��з�Ӧ��һ����ѧ����ʽ_____________________________���÷�Ӧ������Ϊ__________��Ӧ��
(4)�Թܢ��е�����Һ��������____________(�����)
�ټ��� ����ϩ ��Һ̬���� ��Һ̬ϩ��
 ��ҵ����ϵ�д�
��ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ú������ʣ�FeO��Fe2O3���ķ�CuO�Ʊ��������壬�������й��̣�Fe3����pH��5ʱ����ȫ���������з����������
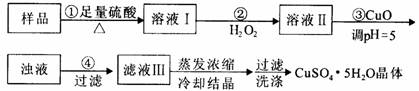
A������CuCO3���CuOҲ�ɵ�����Һ��pH����Ӱ��ʵ����
B��������з�������Ҫ��ӦΪ��H2O2��Fe2����2H����Fe3����2H2O
C��ϴ�Ӿ��壺���˳������©���м�����ˮ����û���壬����Ȼ���º��ظ�2��3��
D������240 mL1mol��L��CuSO4��Һ�������CuSO4·5H2O������Ϊ62.5g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���ǣ� ��
A�� ��
��  ���Ƿ�������
���Ƿ������� ���Ƿ��������Ƿ��㻯����
���Ƿ��������Ƿ��㻯����
B������ʽΪC3H6Cl2���л��ﹲ��5��ͬ���칹�壨�����������칹��
C. ����ʽΪC4H8�л���������4��C—C����
D������ʽΪC2H6O�ĺ������ͼ�Ϸ�����C-H����C-O���������գ��ɴ˿��Ʋ��л���ṹ��ʽΪC2H5-OH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л�����ϩ������ȥ��ϩ�õ������ļ��飬�������ͨ��ʢ�������Լ���ϴ��ƿ ( )
A������ʯ��ˮ��ŨH2SO4 B������KMnO4��Һ��ŨH2SO4
C����ˮ���ռ���Һ��ŨH2SO4 D��ŨH2SO4������KMnO4��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ����������ͨ��30 mLŨ��Ϊ10.00 mol/L����������Ũ��Һ�У���������ʱ�����Һ���γ�NaCl��NaClO��NaClO3������ϵ�������ж���ȷ���ǣ�������
A����NaOH��Ӧ������һ��Ϊ0.15 mol
B��n(Na��)��n(Cl��) ����Ϊ7��3
C������Ӧ��ת�Ƶĵ���Ϊn mol����0.15 < n < 0.25
D��n(NaCl)��n(NaClO)��n(NaClO3)����Ϊ11��2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���Ӧ�У����ڼӳɷ�Ӧ����(����)
A��CH2===CH2��Br2����CH2Br—CH2Br B��CH3CH2OH��3O2 2CO2��3H2O[
2CO2��3H2O[ D��CH4��Cl2
D��CH4��Cl2 CH3Cl��HCl
CH3Cl��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��«���㷺������ʳ�����ɣ驡����������������ѣ��У������ܾ��п����ԡ��ܹ���1mol �û�������Ӧ��Br2��H2����������ֱ��ǣ� )
��«���㷺������ʳ�����ɣ驡����������������ѣ��У������ܾ��п����ԡ��ܹ���1mol �û�������Ӧ��Br2��H2����������ֱ��ǣ� )
A��1mol 1mol B��3.5mol 7mol
C��3.5mol 6mol �� D��6mol 7mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݸ�˹���ɼ��㣺��֪���ʯ��ʯī�ֱ�����������ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C(���ʯ��s)��O2(g)====CO2(g) ��H1����395.41 kJ/mol��
C(ʯī��s)��O2(g)====CO2(g) ��H2����393.51 kJ/mol��
(1)����ʯת��Ϊʯīʱ���Ȼ�ѧ����ʽΪ ��
|
(2)��ȡ���ʯ��ʯī�Ļ�Ͼ��干1 mol��O2����ȫȼ�գ���������ΪQ kJ������ʯ��ʯī���� �ʵ���֮��Ϊ (�ú�Q�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��W��Z�����ֳ����Ķ�����Ԫ�أ���ԭ��
 �뾶��ԭ�������仯����ͼ��ʾ����֪X��һ�ֺ��ص���
�뾶��ԭ�������仯����ͼ��ʾ����֪X��һ�ֺ��ص���
����Ϊ18��������Ϊ10��Y��Neԭ�ӵĺ������������
��1��W�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ķǽ�����
��ͬ��������Ԫ������ǿ��
��1��Wλ��Ԫ�����ڱ��е� ���ڵ� ��
����X�������ӵĽṹʾ��ͼ ��
��2��Z���⻯����廯����ȣ����ȶ����� ��д��ѧʽ����Z���⻯��
���������ȣ��е�ϸߵ��� ��д��ѧʽ����
��3��Y�Ľ�������Mg�Ľ�������ȣ� ��д��ѧʽ���Ľ�����ǿ������ʵ��֤�����ǽ����Ե����ǿ���� ��
��4��д��Y������������Ӧ��ˮ������Z������������Ӧ��ˮ�������Ӧ�Ļ�ѧ��Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com