
ijæĪĶā»ī¶ÆŠ”×éµÄĶ¬Ń§£¬ŃŠ¾æÓĆ¹żĮæµÄŠæÓėÅØĮņĖį·“Ó¦ÖĘČ”¶žŃõ»ÆĮņµÄÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©ŠæÓėÅØĮņĖį·“Ó¦ÖĘČ”¶žŃõ»ÆĮņµÄ»Æѧ·½³ĢŹ½ĪŖ______________________________”£
£Ø2£©ÕāŃłÖĘČ”µÄ¶žŃõ»ÆĮņĘųĢåÖŠæÉÄÜŗ¬ÓŠµÄŌÓÖŹŹĒ__________________£»ŌŅņŹĒ________________________________________________________________________________________________________________________________________________”£
£Ø3£©Ä³Ķ¬Ń§ÓĆĻĀĮŠ×°ÖĆĮ¬½Ó³ÉŅ»ÕūĢ׏µŃé×°ÖĆŅŌŃéÖ¤£Ø2£©µÄÅŠ¶ĻŹĒ·ńÕżČ·£¬Čō°“ĘųĢå“Ó×óµ½ÓŅĮ÷ĻņŹ±£¬ĘųĢåĮ÷¾µÄø÷×°ÖƵ¼¹ÜµÄ±ąŗÅŅĄ“ĪŹĒ________£ØÓĆa”¢b””ĢīŠ“£©”£
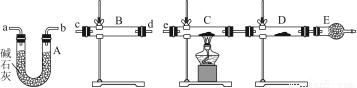
£Ø4£©øł¾Ż£Ø3£©Č·¶ØµÄŹµŃé¹ż³Ģ£¬Ēė½«ŹµŃ鏱ӊ¹Ų×°ÖĆÖŠĖłŹ¢Ņ©Ę·”¢ŹµŃéĻÖĻ󔢽įĀŪ»ņ½āŹĶĢīČėĻĀ±ķ£ŗ
×°ÖĆ | ĖłŹ¢Ņ©Ę· | ŹµŃéĻÖĻó | ½įĀŪ»ņ½āŹĶ |
B |
|
|
|
C | CuO¹ĢĢå |
|
|
£Ø1£©Zn£«2H2SO4£ØÅØ£©=ZnSO4£«SO2”ü£«2H2O
£Ø2£©ĒāĘųŗĶĖ®ÕōĘų””Ėę×Å·“Ó¦µÄ½ųŠŠÅØĮņĖį±»ĻūŗÄ»į±ä³ÉĻ”ĮņĖį£¬ŠæÓėĻ”ĮņĖį·“Ó¦²śÉśĒāĘų£¬Ķ¬Ź±»įÓŠÉŁĮæĖ®ÕōĘų
£Ø3£©cd£Ø»ņdc£©””ab£Ø»ņba£©””e
£Ø4£©
×°ÖĆ | ĖłŹ¢Ņ©Ę· | ŹµŃéĻÖĻó | ½įĀŪ»ņ½āŹĶ |
B | ĪŽĖ®ĮņĖįĶ | ¹ĢĢåÓÉ°×É«±ä³ÉĄ¶É« | SO2ÖŠŗ¬ÓŠĖ®ÕōĘų |
C |
| ¹ĢĢåÓÉŗŚÉ«±ä³ÉŗģÉ« | SO2ÖŠŗ¬ÓŠĒāĘų |
”¾½āĪö”æ£Ø3£©ŅŖ¼ģŃéSO2ÖŠµÄĖ®ÕōĘųŅŌ¼°ĒāĘų£¬ŠčŹ×ĻČÓĆĪŽĖ®ĮņĖįĶ¼ģŃéĖ®ÕōĘų£¬ĒŅÓĆ¼īŹÆ»Ņ³żČ„Ė®ÕōĘų£¬ŌŁ½«Ź£ÓąĘųĢåŅĄ“ĪĶعż×ĘČȵÄŃõ»ÆĶŗĶĪŽĖ®ĮņĖįĶ£¬ĶعżŗŚÉ«Ńõ»ÆĶ±äŗģŗĶĪŽĖ®ĮņĖįĶ±äĄ¶µÄĻÖĻóÖ¤Ć÷ĘųĢåÖŠŗ¬ÓŠĒāĘų£¬×īŗó½«Ź£ÓąĘųĢåÓĆŹ¢ÓŠ¼īŹÆ»ŅµÄøÉŌļ¹ÜĪüŹÕ£¬·ĄÖ¹ĪŪČ¾æÕĘų”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā5»Æѧ·“Ó¦ÖŠµÄÄÜĮæ±ä»ÆĮ·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
CH4”¢H2”¢C¶¼ŹĒÓÅÖŹµÄÄÜŌ“ĪļÖŹ£¬ĖüĆĒČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗ
¢ŁCH4(g)£«2O2(g)=CO2(g)£«2H2O(l)””¦¤H£½£890.3 kJ”¤mol£1£¬
¢Ś2H2(g)£«O2(g)=2H2O(l)””¦¤H£½£571.6 kJ”¤mol£1£¬
¢ŪC(s)£«O2(g)=CO2(g)””¦¤H£½£393.5 kJ”¤mol£1”£
(1)ŌŚÉīŗ£ÖŠ“ęŌŚŅ»ÖÖ¼×ĶéĻø¾ś£¬ĖüĆĒŅĄææĆøŹ¹¼×ĶéÓėO2×÷ÓĆ²śÉśµÄÄÜĮæ“ę»ī£¬¼×ĶéĻø¾śŹ¹1 mol¼×ĶéÉś³ÉCO2ĘųĢåÓėŅŗĢ¬Ė®£¬·Å³öµÄÄÜĮæ________(Ģī”°£¾”±”°£¼”±»ņ”°£½”±)890.3 kJ”£
(2)¼×ĶéÓėCO2æÉÓĆÓŚŗĻ³ÉŗĻ³ÉĘų(Ö÷ŅŖ³É·ÖŹĒŅ»Ńõ»ÆĢ¼ŗĶĒāĘų)£ŗCH4£«CO2=2CO£«2H2£¬1 g CH4ĶźČ«·“Ó¦æÉŹĶ·Å15.46 kJµÄČČĮ棬Ōņ£ŗ
¢ŁĻĀĶ¼ÄܱķŹ¾øĆ·“Ó¦¹ż³ĢÖŠÄÜĮæ±ä»ÆµÄŹĒ________(Ģī×ÖÄø)”£
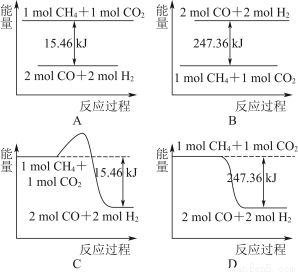
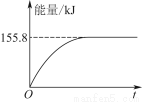
¢ŚČō½«ĪļÖŹµÄĮæ¾łĪŖ1 molµÄCH4ÓėCO2³äČėijŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ĢåĻµ·Å³öµÄČČĮæĖę×ÅŹ±¼äµÄ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņCH4µÄ×Ŗ»ÆĀŹĪŖ________”£
(3)C(s)ÓėH2(g)²»·“Ó¦£¬ĖłŅŌC(s)£«2H2(g)=CH4(g)µÄ·“Ó¦ČČĪŽ·ØÖ±½Ó²āĮ棬µ«ĶعżÉĻŹö·“Ó¦æÉĒó³ö£¬C(s)£«2H2(g)=CH4(g)µÄ·“Ó¦ČȦ¤H£½________”£
(4)ÄæĒ°¶ŌÓŚÉĻŹöČżÖÖĪļÖŹµÄŃŠ¾æŹĒČ¼ĮĻŃŠ¾æµÄÖŲµć£¬ĻĀĮŠ¹ŲÓŚÉĻŹöČżÖÖĪļÖŹµÄŃŠ¾æ·½ĻņÖŠæÉŠŠµÄŹĒ________(Ģī×ÖÄø)”£
A£®Ń°ÕŅÓÅÖŹ“߻ƼĮ£¬Ź¹CO2ÓėH2O·“Ӧɜ³ÉCH4ÓėO2£¬²¢·Å³öČČĮæ
B£®Ń°ÕŅÓÅÖŹ“߻ƼĮ£¬ŌŚ³£ĪĀ³£Ń¹ĻĀŹ¹CO2·Ö½āÉś³ÉĢ¼ÓėO2
C£®Ń°ÕŅÓÅÖŹ“߻ƼĮ£¬ĄūÓĆĢ«ŃōÄÜŹ¹“óĘųÖŠµÄCO2Óėŗ£µ×æŖ²ÉµÄCH4ŗĻ³ÉŗĻ³ÉĘų(CO”¢H2)
D£®½«¹ĢĢ¬Ģ¼ŗĻ³ÉĪŖC60£¬ŅŌC60×÷ĪŖČ¼ĮĻ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā16ĪļÖŹ½į¹¹ÓėŠŌÖŹĮ·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ÓŠA”¢B”¢C”¢D”¢E”¢F 6ÖÖŌŖĖŲ£¬AŹĒÖÜĘŚ±ķÖŠŌ×Ó°ė¾¶×īŠ”µÄŌŖĖŲ£¬BŹĒµēøŗŠŌ×ī“óµÄŌŖĖŲ£¬CµÄ2p¹ģµĄÖŠÓŠ3øöĪ“³É¶ŌµÄµ„µē×Ó£¬FŌ×ÓŗĖĶāµē×ÓŹżŹĒBÓėCŗĖĶāµē×ÓŹżÖ®ŗĶ£¬DŹĒÖ÷×åŌŖĖŲĒŅÓėEĶ¬ÖÜĘŚ£¬EÄÜŠĪ³ÉŗģÉ«£Ø»ņשŗģÉ«£©µÄE2OŗĶŗŚÉ«µÄEOĮ½ÖÖŃõ»ÆĪļ£¬DÓėBæÉŠĪ³ÉĄė×Ó»ÆŗĻĪļ£¬Ę侧°ū½į¹¹ČēĶ¼ĖłŹ¾”£
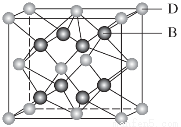
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©EŌŖĖŲŌ×Ó»łĢ¬Ź±µÄµē×ÓÅŲ¼Ź½ĪŖ________________________________________________________________________”£
£Ø2£©FµÄŃõ»ÆĪļFO3·Ö×ÓµÄæռ乹ŠĶĪŖ________”£
£Ø3£©CA3¼«Ņ×ČÜÓŚĖ®£¬ĘäŌŅņÖ÷ŅŖŹĒ________________________________________________________________________”£
ÓėĘä×ī¼ņµ„·Ö×Ó»„ĪŖµČµē×ÓĢåµÄŃōĄė×ÓĪŖ________”£
£Ø4£©ĻĀĮŠ·Ö×Ó½į¹¹Ķ¼ÖŠµÄ”° ”±±ķŹ¾Ļą¹ŲŌŖĖŲµÄŌ×ÓÖŠ³żČ„×īĶā²ćµē×ӵď£Óą²æ·Ö£¬”°
”±±ķŹ¾Ļą¹ŲŌŖĖŲµÄŌ×ÓÖŠ³żČ„×īĶā²ćµē×ӵď£Óą²æ·Ö£¬”° ”±±ķŹ¾ĒāŌ×Ó£¬Š”ŗŚµć”°
”±±ķŹ¾ĒāŌ×Ó£¬Š”ŗŚµć”° ”±±ķŹ¾Ć»ÓŠŠĪ³É¹²¼Ū¼üµÄ×īĶā²ćµē×Ó£¬¶ĢĻß±ķŹ¾¹²¼Ū¼ü”£
”±±ķŹ¾Ć»ÓŠŠĪ³É¹²¼Ū¼üµÄ×īĶā²ćµē×Ó£¬¶ĢĻß±ķŹ¾¹²¼Ū¼ü”£
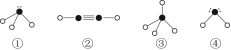
ŌņŌŚŅŌÉĻ·Ö×ÓÖŠ£¬ÖŠŠÄŌ×Ó²ÉÓĆsp3ŌӻƊĪ³É»Æѧ¼üµÄŹĒ________£ØĢīŠ“ŠņŗÅ£©£»ŌŚ¢ŚµÄ·Ö×ÓÖŠÓŠ________øö¦Ņ¼üŗĶ________øö¦Š¼ü”£
£Ø5£©“ÓĶ¼ÖŠæÉŅŌ擳ö£¬DÓėBŠĪ³ÉµÄĄė×Ó»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ________”£
£Ø6£©Ķ¼ÖŠ£¬ČōDÓėBµÄĄė×Ó»ÆŗĻĪļ¾§ĢåµÄĆܶČĪŖa g”¤cm£3£¬Ōņ¾§°ūµÄĢå»żŹĒ________________________________________________________________________£ØŠ“³ö±ķ“ļŹ½¼“æÉ£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā14×ŪŗĻŹµŃéÓėĢ½¾æĮ·Ļ°¾ķB£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠøł¾ŻŹµŃé²Ł×÷ŗĶĻÖĻóĖłµĆ³öµÄ½įĀŪÕżČ·µÄŹĒ£Ø””””£©
Ń”ĻīŹµŃé²Ł×÷ŹµŃéĻÖĻó½į ĀŪ
A½«ÉŁĮæFe£ØNO3£©2ŹŌŃł¼ÓĖ®Čܽā£¬µĪ¼ÓĻ”H2SO4Ėį»Æ£¬ŌŁµĪ¼ÓKSCNČÜŅŗČÜŅŗ±ä³ÉŗģÉ« Fe£ØNO3£©2ŹŌŃłŅѱäÖŹ
B½«ÉŁĮæijĪŽÉ«ĘųĢåĶØČė³ĪĒåŹÆ»ŅĖ®³öĻÖ°×É«³ĮµķøĆĘųĢåŅ»¶ØŹĒCO2
C·Ö±š²ā¶Ø³£ĪĀĻĀ0.1 mol”¤L£1 Na2SiO3ČÜŅŗŗĶNa2CO3ČÜŅŗµÄpHpH£ŗNa2SiO3> Na2CO3 ·Ē½šŹōŠŌ£ŗSi>C
DĻņÅØ¶Č¾łĪŖ0.1 mol”¤L£1 NaClŗĶNaI»ģŗĻČÜŅŗÖŠ£¬µĪ¼ÓÉŁĮæAgNO3ČÜŅŗ³öĻÖ»ĘÉ«³Įµķ Ksp£ØAgCl£©£¾Ksp£ØAgI£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā14×ŪŗĻŹµŃéÓėĢ½¾æĮ·Ļ°¾ķA£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠÓŠ¹ŲŹµŃé×°ÖĆ½ųŠŠµÄĻąÓ¦ŹµŃ飬ÄÜ“ļµ½ŹµŃéÄæµÄµÄŹĒ£Ø””””£©
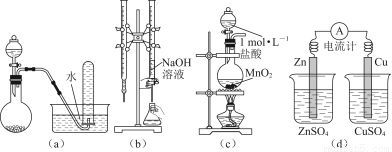
A£®ÓĆČēĶ¼£Øa£©ĖłŹ¾×°ÖĆ½ųŠŠĻ”ĻõĖįÓėĶµÄ·“Ó¦ÖĘČ”²¢ŹÕ¼ÆNO
B£®ÓĆĶ¼£Øb£©ĖłŹ¾×°ÖĆ½ųŠŠÓĆŅŃÖŖÅØ¶ČµÄĒāŃõ»ÆÄĘČÜŅŗ²ā¶ØŃĪĖįÅØ¶ČµÄŹµŃé
C£®ÓĆĶ¼£Øc£©ĖłŹ¾×°ÖĆÖĘȔɣĮæCl2
D£®ÓĆĶ¼£Ød£©ĖłŹ¾×°ÖĆ¼ģŃéµēĮ÷µÄ·½Ļņ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā13»ÆѧŹµŃ黳“”Į·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
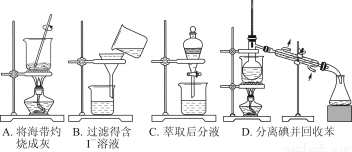
“Óŗ£“ųÖŠĢįČ”µāµÄŹµŃé¹ż³ĢÖŠ£¬Éę¼°ĻĀĮŠ²Ł×÷£¬ĘäÖŠÕżČ·µÄŹĒ£Ø””””£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā13»ÆѧŹµŃ黳“”Į·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ĻĀĮŠŠšŹöÖŠ£¬ÕżČ·µÄŹĒ£Ø””””£©
A£®³żČ„FeCl2ČÜŅŗÖŠÉŁĮæµÄFeBr2£¬¼ÓČėŹŹĮæĀČĖ®
B£®½«CO2ĶØČėBaCl2ČÜŅŗÖŠÖĮ±„ŗĶ£¬ĪŽ³Įµķ²śÉś£¬ŌŁĶØČėSO2£¬²śÉś³Įµķ
C£®½«Cl2ĶØČėµķ·ŪKIČÜŅŗ£¬ČÜŅŗ±äĄ¶
D£®ŌŚĻ”ĮņĖįÖŠ¼ÓČėĶ·Ū£¬Ķ·Ū²»Čܽā£»ŌŁ¼ÓČėKNO3¹ĢĢ壬Ķ·ŪČŌ²»Čܽā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖø“Ļ°ĻŽŹ±¼Æѵ ×ØĢā11½šŹōŌŖĖŲµ„ÖŹ¼°»ÆŗĻĪļĮ·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ĮāŠææóµÄÖ÷ŅŖ³É·ÖŹĒĢ¼ĖįŠæ£¬»¹ŗ¬ÓŠÉŁĮæµÄFe2O3”¢FeO”¢CuOµČŌÓÖŹ”£ČēĶ¼ŹĒŅŌĮāŠææóĪŖŌĮĻÖĘČ”ĒįÖŹŃõ»ÆŠæµÄŅ»ÖÖ¹¤ŅÕĮ÷³Ģ£¬øĆĮ÷³Ģ»¹æÉŅŌµĆµ½Į½ÖÖø±²śĘ·”Ŗ”Ŗŗ£ĆąĶŗĶĢśŗģ”£
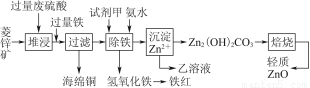
Ēė½įŗĻĻĀ±ķŹż¾Ż£¬»Ų“šĪŹĢā£ŗ
Ąė×Ó | æŖŹ¼³ĮµķŹ±µÄpH | ĶźČ«³ĮµķŹ±µÄpH |
Fe2£« | 6.3 | 9.7 |
Fe3£« | 1.5 | 3.2 |
Zn2£« | 6.2 | 8.0 |
£Ø1£©ĻĀĮŠŹŌ¼ĮÖŠ£¬________£ØĢī“śŗÅ£©æÉ×÷ĪŖŹŌ¼Į¼×µÄŹ×Ń””£
A£®KMnO4”” B£®Cl2”” C£®H2O2”” D£®ÅØĻõĖį
øł¾ŻĖłŃ”ŹŌ¼ĮŠ“³öÓėÖ®·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ________________________________________________________________________________________________________________________________________________”£
£Ø2£©ĒāŃõ»ÆĢś¹ĢĢå»įĪüø½Ņ»Š©SO42-£¬ČēŗĪĻ“µÓFe£ØOH£©3¹ĢĢåŅŌ¼°ČēŗĪÅŠ¶ĻŹĒ·ńĻ“µÓøɾ»£æ
Ļ“µÓ·½·Ø£ŗ______________________________________________________________£¬ÅŠ¶ĻŹĒ·ńĻ“µÓøɾ»µÄ·½·ØŹĒ_______________________________________”£
£Ø3£©³żĢś²½ÖčÖŠ¼ÓČė°±Ė®µÄÄæµÄŹĒµ÷½ŚČÜŅŗµÄpH£¬Ę䏏ŅĖµÄpH·¶Ī§ŹĒ______________£»µ÷½ŚČÜŅŗpHŹ±£¬³żĮĖ°±Ė®Ķā£¬»¹æÉŅŌ¼ÓČėĻĀĮŠĪļÖŹÖŠµÄ________”£
a£®Zn”” b£®ZnO”” c£®Zn£ØOH£©2”” d£®CuO
£Ø4£©¾²ā¶ØŅŅČÜŅŗÖŠČŌŗ¬ÓŠÉŁĮæµÄFe3£«ŗĶZn2£«”£Čōc£ØFe3£«£©ĪŖ4.0”Į10£17 mol”¤L£1£¬Ōņc£ØZn2£«£©ĪŖ______________ mol”¤L£1”££ØŅŃÖŖKsp[Fe£ØOH£©3]£½4.0”Į10£38£¬Ksp[Zn£ØOH£©2]£½1.2”Į10£17£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014øßæ¼»Æѧ¶žĀÖ×ØĢāĶ»ĘĘ ×ØĢāŹ®µē½āÖŹČÜŅŗĮ·Ļ°¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
ŹŅĪĀĻĀ£¬½«Ņ»ŌŖĖįHAµÄČÜŅŗŗĶKOHČÜŅŗµČĢå»ż»ģŗĻ(ŗöĀŌĢå»ż±ä»Æ)£¬ŹµŃ鏿¾ŻČēĻĀ±ķ£ŗ
ŹµŃ鱹ŗÅ | ĘšŹ¼ÅضČ/mol”¤L£1 | ·“Ó¦ŗóČÜŅŗµÄpH | |
c(HA) | c(KOH) | ||
¢Ł | 0.1 | 0.1 | 9 |
¢Ś | x | 0.2 | 7 |
ĻĀĮŠÅŠ¶Ļ²»ÕżČ·µÄŹĒ(””””)
A£®ŹµŃé¢Ł·“Ó¦ŗóµÄČÜŅŗÖŠ£ŗc(K£«)£¾c(A£)£¾c(OH£)£¾c(H£«)
B£®ŹµŃé¢Ł·“Ó¦ŗóµÄČÜŅŗÖŠ£ŗc(OH£)£½c(K£«)£c(A£)£½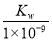 mol”¤L£1
mol”¤L£1
C£®ŹµŃé¢Ś·“Ó¦ŗóµÄČÜŅŗÖŠ£ŗc(A£)£«c(HA)£¾0.1 mol”¤L£1
D£®ŹµŃé¢Ś·“Ó¦ŗóµÄČÜŅŗÖŠ£ŗc(K£«)£½c(A£)£¾c(OH£)£½c(H£«)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com