
����Ŀ����֪ͭ�������A�ṹ��ͼ����ش��������⣺

(1)д����̬Cu����Χ�����Ų�ʽ_____________________________________��
(2)���就�������(H2NCH2COO��)���ȷֽ�ɲ���CO2��N2��N2��������������Ŀ֮����__________��N2O��CO2��Ϊ�ȵ����壬��N2O�ĵ���ʽΪ____________��
(3)��Cu���£��״��ɱ�����Ϊ��ȩ����ȩ������HCO�ļ���_____(ѡ������������������������С����)120������ȩ����ˮ�γ������������ͼ�б�ʾ����_____��

(4)������������ͼ����ʯ�ṹ���ƣ��dz�Ӳ���ϡ���������������B-N��������ԭ����֮��Ϊ___________��

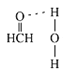
(5)Cu����Ķѻ���ʽ��ͼ��ʾ����Cuԭ�Ӱ뾶Ϊa��������Cuԭ�ӵ���λ��Ϊ________������Ŀռ�������Ϊ______��(��֪:![]() ����ʽ����������)
����ʽ����������)

���𰸡�3d104s1 1��2 ![]() ����
����  4��1 12 74.76%
4��1 12 74.76%
��������
(1)Cuԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
(2)����N2�ĽṹʽΪN��N�ж�������������Ŀ֮�ȣ��ȵ�����Ľṹ���ƣ����ݶ�����̼����ʽ��дN2O�ĵ���ʽ��
(3)��ȩ��C��sp2�ӻ�����ƽ�������Σ���Ϲ¶Ե�����ɼ����ӶԵ����÷����жϼ��ǵĴ�С����ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ������
(4)�þ�����Nԭ���ھ����ڲ���ÿ��Nԭ���γ�4��B-N���������ж�B-N��������Bԭ�Ӹ���֮�ȣ�
(5)Cu����Ķѻ���ʽ�������������ܶѻ����ݴ��ж���λ�������������ĶԽ��߳���4r���������������ⳤ�;�������������ݾ����Ľṹ�����Cuԭ�ӵĸ����������ݾ���Ŀռ�������=![]() ��100%���㡣
��100%���㡣
(1)Cuԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s1�����Ի�̬Cu����Χ�����Ų�ʽΪ��3d104s1���ʴ�Ϊ��3d104s1��
(2)N2�ĽṹʽΪN��N��������������Ŀ֮��Ϊ1��2���ȵ�����Ľṹ���ƣ����ݶ�����̼����ʽ������дN2O�ĵ���ʽΪ![]() ���ʴ�Ϊ��1��2��
���ʴ�Ϊ��1��2��![]() ��
��
(3)��ȩ��C��sp2�ӻ�����ƽ�������Σ�Cԭ�����������ڲ���C-H��C-H���н���������120�㣬���������ʻ����ŶԵ��ӵ��ų⣬ʵ�ʼ���Ӧ����С��120�㣬���Լ�ȩ������HCO�ļ��Ǵ���120�㣻��ȩ�����е�Oԭ�Ӻ�ˮ�����е�Hԭ�����γ�������������ʾΪ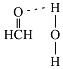 ���ʴ�Ϊ�����ڣ�
���ʴ�Ϊ�����ڣ� ��
��
(4)�þ�����Nԭ���ھ����ڲ���������4��ÿ��Nԭ���γ�4��B-N����B-N������Ϊ16��Bԭ��λ�ھ�����������ģ�����=8��![]() +6��
+6��![]() =4����B-N����Bԭ�Ӹ���֮��Ϊ16��4=4��1���ʴ�Ϊ��4��1��
=4����B-N����Bԭ�Ӹ���֮��Ϊ16��4=4��1���ʴ�Ϊ��4��1��
(5)Cu����Ķѻ���ʽ�������������ܶѻ���������λ����12����ͼ��֪�����������ĶԽ��߳���4a��������������ⳤ=2![]() a��1��Cu�������
a��1��Cu�������![]() ��a3��������Cuԭ�ӵĸ���=8��
��a3��������Cuԭ�ӵĸ���=8��![]() +6��
+6��![]() =4�������Ծ���Ŀռ�������Ϊ
=4�������Ծ���Ŀռ�������Ϊ![]() ��100%=74.76%���ʴ�Ϊ��12��74.76%��
��100%=74.76%���ʴ�Ϊ��12��74.76%��


 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������﮿�����������ܶȸߡ��ɱ��ͣ�����Ϊδ���綯�����Ķ���Դ���乤��ԭ������ͼ��ʾ�������йظõ�ص�˵����ȷ����

A. ���ʱ�������Ϊ����
B. �ŵ�ʱ������������Χ����LiOH
C. �ŵ�ʱ��ÿ����22.4LO2��ת��4mol����
D. �ŵ�ͳ��ʱ��Li+Ǩ�Ʒ����෴
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪����ʱHClO��Ka=3.0��10-8��HF��Ka=3.5��10-4���ֽ�pH���������ͬ�Ĵ�������������Һ�ֱ������ˮϡ�ͣ�pH����Һ����ı仯��ͼ��ʾ������������ȷ����

A. ����IΪ������ϡ��ʱpH�仯����
B. ȡa�����������Һ���к���ͬ�������ͬŨ�ȵ�NaOH��Һ�����Ĵ�����������С
C. a��ʱ������������ͬ��С��п������ʱ������ᷴӦ�����ʴ�
D. b����Һ��ˮ�ĵ���̶ȱ�c����Һ��ˮ�ĵ���̶�С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��Ka(CH3COOH)=1.7��10-5�����¶��£���0.1mol/L�Ĵ���ζ�10.00 mL0.1mol/L�ļ�MOH���ζ������м����������(V) ����Һ��lg[c (H+)/c(OH-)]�Ĺ�ϵ��ͼ��ʾ��V=0ʱ��

lg[c (H+)/c(OH-)]=-12��������˵����ȷ������ ��
A. MOH�ĵ��뷽��ʽΪMOH![]() M++OH-
M++OH-
B. a ��:V(CH3COOH)= 10.00mL
C. b��:c(CH3COO-)>c(H+ )>c(M+)>c(OH-)
D. 25��ʱ��CH3COO-��ˮ��ƽ�ⳣ��Ϊ(10/17)��10-9
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(HN3)������(NaN3��NH4N3��CuN3��) �����б�ը�ԣ�����Ͼ�������ѧ�����ɽ����Ŷӳɹ��ϳɳ�PHAC���仯ѧʽΪ(N3)3 (NH4)4Cl���ش����⣺
(1) PHAC����N3���Ļ��ϼ�Ϊ_____________��N2F2�ĵ���ʽΪ_____________��
(2)������ȫ������NaN3�ɷ������з�Ӧ��
NaN3(s)=Na(s)+![]() N2(g) ��H1
N2(g) ��H1
2NaN3(s)+CuO(s)=Na2O(s)+3N2(g)+Cu(s) ��H2
��ӦCuO(s)+2Na(s)= Na2O(s)+ Cu(s) ��H=_______(�á�H1�͡�H2��ʾ)
(3) 25�棬��1molNH4N3(s)Ͷ��һ2L�ĺ����ܱ������У�0.5min��Ӧ�ﵽƽ�⣬������ɵ����ֵ��ʵ����ʵ���֮��Ϊ1.6mol����NH4N3��ƽ��ת����Ϊ________��25��ʱ�÷�Ӧ��ƽ�ⳣ��K=__________��
(4) �������(HN3)������ˮ������������������
�� HN3��ˮ��Һ�еĵ��뷽��ʽΪ_____________��
�� 0.1mol��L-1��HN3��Һ��0.1mol��L-1��NaN3�������ϣ������Һ�и�����Ũ���д�С��˳��Ϊ_________________��
�� ��֪T��ʱ��Ksp(CuN3)=5.0��10-9��Ksp(Cu2S)=2.5��10-48������ͬ�¶��·�Ӧ��
Cu2S(s)+2N3-(aq) ![]() 2CuN3(s)+S2-(aq)��ƽ�ⳣ��K=_______________��
2CuN3(s)+S2-(aq)��ƽ�ⳣ��K=_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����˷�Ӧ�Ǻϳɷ����廯�����һ����Ҫ�������л���a(��RΪ����)�ͱ�ͨ�����˷�Ӧ�ϳ�b�Ĺ�������(��С���Ӳ�����ȥ)

����˵���������
A. һ�������±���������Ӧ�IJ���֮һ����ϩ���ݣ�2��3�ݼ���![]() ��Ϊͬ���칹��
��Ϊͬ���칹��
B. b�Ķ��ȴ��ﳬ������
C. RΪC5H11ʱ��a�Ľṹ��3��
D. RΪC4H9ʱ��1molb�ӳ�����C10H20������Ҫ3molH2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о���ѧ��Ӧʱ����Ҫ�������ʱ仯�������仯����Ҫ��ע��Ӧ�Ŀ������ȡ��ش���������:
(1)NH3��ԭNO����Ҫ�����������������䷴Ӧ������������ϵ��ͼ��ʾ

����ͼ����Ϊ�ı��˷�Ӧ��������Ӧ�Ļ�ܣ�b_______(����������������������)a��
��������Ӧ���Ȼ�ѧ����ʽ�ɱ�ʾΪ��Ӧ�����������H��______(��E1��E2�Ĵ���ʽ��ʾ)��
���о����֣�һ�������µ�������Ӧ���̿�����ͼ��ʾ������������ԭ��Ӧ�����ʵ����ã�NOΪ_______���������ܷ�Ӧ�Ļ�ѧ����ʽΪ_______________��

(2)һ���¶��£�����ͬ���ʵ�����H2O(g)��CO�ֱ�ͨ���ݻ�Ϊ1L�ĺ����������У����з�ӦH2O(g)��CO(g)![]() CO2(g)��H2(g)���õ������ʾ����������
CO2(g)��H2(g)���õ������ʾ����������
������ | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ��ʱ��/min | ||
H2O(g) | CO(g) | CO(g) | H2(g) | |||
1 | 650 | 2.0 | 4.0 | 3.0 | 1.0 | 5 |
2 | 900 | 1.0 | 2.0 | 1.8 | 0.2 | 4 |
3 | 900 | a | b | c | d | t |
��4mim�ڣ�ʵ��2��v(CO2)��______�� 900��ʱ����Ӧ��ƽ�ⳣ��Ϊ______�������¶�ʱ��ƽ�ⳣ����________(��������������С������������)��
��650��ʱ�����ڴ������г���2.0 mol H2O(g)��1.0molCO(g)��1.0 mol CO2(g)�� xmol H2(g)��Ҫʹ��Ӧ�ڿ�ʼʱ������Ӧ������У���xӦ�����������__________��
��a��2.0��b��1.0����ƽ��ʱʵ��2��H2O(g)��ʵ��3��CO(g)��ת����(a)�Ĺ�ϵΪa(H2O) _______ (�������������ɣ���)a(CO)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[��ѧ-ѡ��3:���ʽṹ������]���軯�أ���ѧʽΪK3[Fe(CN)6]����ҪӦ������ҩ����ơ���ֽ�����������ȹ�ҵ�������շֽ�����KCN��FeC2��N2��(CN)2�����ʡ�
��1����̬Kԭ�Ӻ�������Ų���дʽΪ___________�� K3[Fe(CN)6]�����漰��Ԫ�صĻ�̬ԭ�Ӻ���δ�ɶԵ�����������_________����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________��
��2��(CN)2�����д���̼̼����������ЦҼ���м���Ŀ֮��Ϊ_______��KCN���������ÿ�����HCN��HCN������ԭ�ӵ��ӻ��������Ϊ_________��
��3��CO�������Fe�γ�Fe(CO)5���û������۵�Ϊ253 K���е�ΪΪ376 K�����������_____���塣
��4����ͼ�ǽ������ʳ��������ֶѻ���ʽ�ľ���ģ�͡�

�������ɵ���a�ѻ���ʽ.��ԭ�ӵ���λ��Ϊ_____���þ�����ԭ�������ռ��������ı�ֵΪ____(�ú��е�������ʽ��ʾ)��
�ڳ����Ľ��������ɵ���b�ѻ���ʽ����ԭ�ӵİ뾶Ϊr pm�����侧���ܶ�Ϊ_____g��cm-3(�ú���r��NA��������ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�����50 mL 0.1 mol��L-1 NH4HSO4��Һ�еμ�0.05 mol��L-1 NaOH��Һ���õ���Һ��pH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ(����μӹ���������������)������˵����ȷ����

A��b����Һ������Ũ���ɴ�С��˳��Ϊ��c(Na+)��c(SO![]() )��c(NH
)��c(NH![]() )��c(H+)��c(OH-)
)��c(H+)��c(OH-)
B��ͼ��b��c��d������Һ��ˮ�ĵ���̶�������c��
C��b����μ�NaOH��Һ�����У�NH3��H2O�ĵ���̶���С
D��pH=7ʱ����Һ��c(H+)+c(Na+)+c(NH![]() )=c(SO
)=c(SO![]() )+c(OH-)
)+c(OH-)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com