
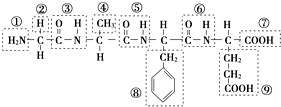
��12�֣��� ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺

��1���û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�
��2��д���û�����ˮ�����ɵ�����һ�ְ�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
�� ʳƷ��ȫ��ϵ�Ź������ƣ�Ӱ��ʳƷ��ȫ�����غܶࡣ
��3����ƫ������ϩ(? ?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
(4)����ֲ�����е�������[CH3(CH2)4��CH===CH��CH2��CH===CH��(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����________��������ţ�
A������ʽΪC18H34O2
B��һ���������������(������)����������Ӧ
C���ܺ�NaOH��Һ��Ӧ
D����ʹ����KMnO4��Һ��ɫ
(5)�پ��м״�(CH3OH)�������꣬��д��Na�ͼ״���Ӧ�Ļ�ѧ����ʽ��______________________��
��6���������յ�ˮ������������ǡ������ʵ��֤�������Ѿ�ȫ��ˮ�⣬д������������ͽ��ۣ�______________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺
��1��ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺
| ϡ���� |
| ϡ���� |
| ϡ���� |
| ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ��ˮ��ѧ�߶���ѧ���ĵ����Ի�ѧ�Ծ� ���ͣ������
��12�֣���ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺
��1���û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�
��2��д���û�����ˮ�����ɵ�����һ�ְ�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
�� ʳƷ��ȫ��ϵ�Ź������ƣ�Ӱ��ʳƷ��ȫ�����غܶࡣ
��3����ƫ������ϩ(? ?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
?)���г�ǿ������ܣ�����Ϊ����ʳƷ�İ�װ���ϡ�������________(д�ṹ��ʽ)�����Ӿ۷�Ӧ���ɵġ�
(4)����ֲ�����е�������[CH3(CH2)4��CH===CH��CH2��CH===CH��(CH2)7COOH]�����ܵ͡����й����������˵���У���ȷ����________��������ţ�
| A������ʽΪC18H34O2 |
| B��һ���������������(������)����������Ӧ |
| C���ܺ�NaOH��Һ��Ӧ |
| D����ʹ����KMnO4��Һ��ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����һ�и߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
������16�֣�
(1)ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺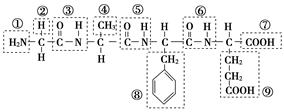
�û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�д���û�����ˮ�����ɵİ�����Ľṹ��ʽ(��дһ��)��________����д���˰�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
(2)ij�л��ﺬ��C��N��H��O����Ԫ�أ���ͼΪ���л�������ģ�͡�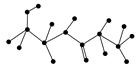
�ٸ��л���Ļ�ѧʽ______________________________��
�ṹ��ʽ_______________________________________________ _______��
_______��
�ڸ��л�����ܷ����Ļ�ѧ��Ӧ��(����)________��
a��ˮ�⡡b���Ӿ�c��ȡ�� d����ȥe������
�۸��л����ˮ�ⷴӦ�Ļ�ѧ����ʽ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
������16�֣�
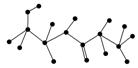
(1)ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺
�û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�д���û�����ˮ�����ɵİ�����Ľṹ��ʽ(��дһ��)��________����д���˰�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
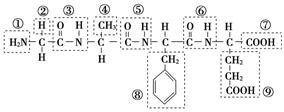
(2)ij�л��ﺬ��C��N��H��O����Ԫ�أ���ͼΪ���л�������ģ�͡�
�ٸ��л���Ļ�ѧʽ______________________________��
�ṹ��ʽ______________________________________________________��
�ڸ��л�����ܷ����Ļ�ѧ��Ӧ��(����)________��
a��ˮ�⡡b���Ӿ�c��ȡ�� d����ȥe������
�۸��л����ˮ�ⷴӦ�Ļ�ѧ����ʽ__________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com