
| ʵ���Լ� | ���� | ���� | ���� | �йط���ʽ |
| ______ | ______ | ______ | ______ | ______ |
| ʵ���Լ� | ���� | ���� | ���� | �йط���ʽ |
| �Ȼ�����Һ������ | ȡ�������Ⱥ�Ĺ������������ˮ�����Һ��ȡ��Һ�������Թ��У������м�ϡ���������Һ�м����Ȼ�����Һ | �г���������ζ�����������ɣ��������ᱵ��ɫ�� | ֤���ֽ�����к������ƺ������� | S2-+2H+=H2S����Ba2++SO42-=BaSO4�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
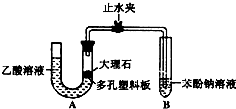 ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ��������������һЩʵ�飺
ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ��������������һЩʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007��1�½���ʡ�����ѧ������ѧ��ĩ�ۺ���ϰ�����˽� ���ͣ�058
| |||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
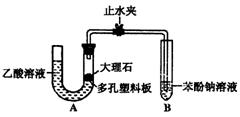 ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ����������
ijУ��ѧ��ȤС��ѧ��Ϊ��̽�����ᡢ̼��ͱ��ӵ�����ǿ������Уѧ������װ����ͼ��ʵ��װ�ã��г���������ȥ����������
����һЩʵ�飺
��1��ij�����װ��A��������ʱ���ȹر�ֹˮ
�У��������U���ڼ�ˮ�������Һ�������
��Һ�棬����һ��ʱ�����U������Һ�������仯�������������á�����Ϊ��ѧ���IJ�����ȷ��� (���ȷ������)
��2������ʯ��������Һ��Ӧ�Ļ�ѧ����ʽΪ
��3��װ��A�з�Ӧ����������ͨ�뱽������Һ�У�ʵ������Ϊ ����Ӧ�����ӷ���ʽΪ ��
��4����ѧ����Ϊ��2���е�ʵ����������֤��̼������Աȱ���ǿ������������ ���Ľ���װ�õķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���㽭ʡ��������У�����߶����ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com