
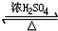 CH3COOCH2CH3+H2O���÷�Ӧ������������ȡ������Ӧ��
CH3COOCH2CH3+H2O���÷�Ӧ������������ȡ������Ӧ�� ��
��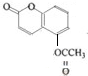 +4NaOH$\stackrel{��}{��}$$��_{��}^{NaOH}$CH3COONa+
+4NaOH$\stackrel{��}{��}$$��_{��}^{NaOH}$CH3COONa+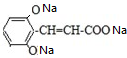 +2H2O��
+2H2O�� ���� ��1��������A��C11H10O5��������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C��˵��A�к���������B�ķ���ʽΪC2H4O2��������ֻ��һ�������ţ�����B��CH3COOH��������Ҵ��ܷ���������Ӧ����DΪCH3COOC2H5��
��2��B��ͬ���칹���ܷ���������Ӧ������ȩ��-CHO��Ϊ�����γɵ�����
��3��C�Ƿ��㻯���˵��C�к��б�������Է�������Ϊ180��������Ԫ�ص���������Ϊ$\frac{16}{45}$����Oԭ�Ӹ���Ϊ$\frac{180��\frac{16}{45}}{16}$=4��A��C11H10O5���к���������B�ķ���ʽΪC2H4O2����C�ķ���ʽ��C9H8O4��
��4��C�ķ�����������ȡ����������һ��ȡ������֧��������̼��������Һ��Ӧ�ų����壬˵�������Ȼ���������ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ţ���Ϸ���ʽ�жϺ���̼̼˫������������ȡ������ͬ�����ݷ���ʽ��֪Ϊ-OH��������һ�ȴ������2��˵�������Ͻ�����2����ԭ�ӣ���C�Ľṹ��ʽ ��
��
��5������2��-OH������λ��λ����λ���ƶ�-CH=CHCOOHȷ��C��ͬ���칹����Ŀ��
��6��A+H2O$��_{��}^{NaOH}$B+C��B�ķ���ʽΪC2H4O2��C�ķ���ʽ��C9H8O4�����ݣ�4��C�Ľṹ��ʽ��д��
��� �⣺��1��������A��C11H10O5��������������Һ�м��ȷ�Ӧ�����ữ�ɵõ�������B��C��˵��A�к���������B�ķ���ʽΪC2H4O2��������ֻ��һ�������ţ�����B��CH3COOH��������Ҵ��ܷ���������Ӧ����DΪCH3COOC2H5����Ӧ����ʽΪ��CH3COOH+CH3CH2OH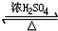 CH3COOCH2CH3+H2O������������ȡ������Ӧ��
CH3COOCH2CH3+H2O������������ȡ������Ӧ��
�ʴ�Ϊ��CH3COOH��CH3COOH+CH3CH2OH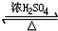 CH3COOCH2CH3+H2O��������ȡ������Ӧ��
CH3COOCH2CH3+H2O��������ȡ������Ӧ��
��2��B��ͬ���칹���ܷ���������Ӧ������ȩ��-CHO��Ϊ�����γɵ�������ṹ��ʽΪHOCH2CHO��HCOOCH3��
�ʴ�Ϊ��HOCH2CHO��HCOOCH3��
��3��C�Ƿ��㻯���˵��C�к��б�������Է�������Ϊ180��������Ԫ�ص���������Ϊ$\frac{16}{45}$����Oԭ�Ӹ���Ϊ$\frac{180��\frac{16}{45}}{16}$=4��A��C11H10O5���к���������B�ķ���ʽΪC2H4O2��B��CH3COOH��A+H2O$��_{��}^{NaOH}$B+C����C�ķ���ʽ��C9H8O4��
�ʴ�Ϊ��C9H8O4��
��4��C�ķ�����������ȡ����������һ��ȡ������֧��������̼��������Һ��Ӧ�ų����壬˵�������Ȼ���������ʹ������Ȼ�̼��Һ��ɫ�Ĺ����ţ���Ϸ���ʽ�жϺ���̼̼˫������������ȡ������ͬ�����ݷ���ʽ��֪Ϊ-OH��������һ�ȴ������2��˵�������Ͻ�����2����ԭ�ӣ���C�Ľṹ��ʽ�� ��
��
�ʴ�Ϊ�� ��
��
��5����2��-OH������λ��-CH=CHCOOH��2��λ�ã���2��-OH���ڼ�λ��-CH=CHCOOH��3��λ�ã�����C���ڣ�����2��-OH���ڶ�λ��-CH=CHCOOH��1��λ�ã��ʲ���C���ڹ���5�֣�
�ʴ�Ϊ��5��
��6��B��CH3COOH��C�Ľṹ��ʽ ���ۺ�����������֪��A�Ľṹ��ʽΪ
���ۺ�����������֪��A�Ľṹ��ʽΪ ��A+H2O��$��_{��}^{NaOH}$B+C��A������NaOH��Һ�м��ȷ�Ӧ�Ļ�ѧ����ʽΪ
��A+H2O��$��_{��}^{NaOH}$B+C��A������NaOH��Һ�м��ȷ�Ӧ�Ļ�ѧ����ʽΪ +4NaOH$\stackrel{��}{��}$$��_{��}^{NaOH}$CH3COONa+
+4NaOH$\stackrel{��}{��}$$��_{��}^{NaOH}$CH3COONa+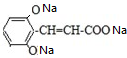 +2H2O��
+2H2O��
�ʴ�Ϊ�� +4NaOH$\stackrel{��}{��}$$��_{��}^{NaOH}$CH3COONa+
+4NaOH$\stackrel{��}{��}$$��_{��}^{NaOH}$CH3COONa+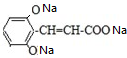 +2H2O��
+2H2O��
���� ���⿼���л�����ƶϣ�Ϊ�߿��������ͣ������ڿ���ѧ���ۺ����û�ѧ֪ʶ�������ͷ�����������������������Ϣ��ȷ�ƶ�C�Ľṹ��ʽΪ������Ĺؼ���ע������л�������ŵ����ʣ���Ŀ�Ѷ��еȣ�


 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.44 s | B�� | 2 s | C�� | 0.33 s | D�� | 1 s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
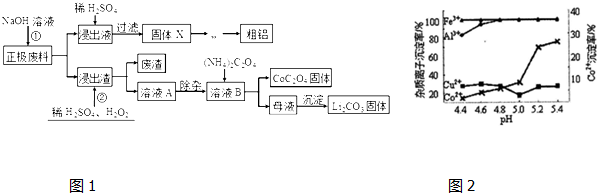
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ��ɺͽṹ��Ϣ |
| a | ����A�Ķ�Ԫ���ӻ����� |
| b | ���й��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ��ѧ���ΪAFD |
 ��c�ĽṹʽΪH-O-Cl��
��c�ĽṹʽΪH-O-Cl���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��صĵ��Һ��ΪLiClˮ��Һ | |
| B�� | �õ�طŵ�ʱ������������ԭ��Ӧ | |
| C�� | �ŵ�ʱ������ӦʽΪ��2SOCl2+4e-=4Cl-+S+SO2 | |
| D�� | �ŵ�ʱ���ӴӸ��������·�����������ٴ���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��H+��SO42-��CO32- | B�� | Na+��Ca2+��SO42-��NO3- | ||
| C�� | Ag+��Mg2+��Cl-��S2- | D�� | Na+��Cu2+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com