
(12·Ö)øł¾ŻŅŖĒó»Ų“šĻą¹ŲĪŹĢā£ŗ
(1) ³żČ„ĻĀĮŠĪļÖŹÖŠĖł»ģÓŠµÄÉŁĮæŌÓÖŹ(ĄØŗÅÄŚĪŖŌÓÖŹ)£¬Š“³öÓŠ¹ŲµÄ·“Ó¦·½³ĢŹ½”£
¢ŁĶ·Ū (ĀĮ·Ū) £»
¢ŚFeCl3 ČÜŅŗ(FeCl2 ) £»
¢ŪN2 (O2) ____________________________________________________”£
(2)ĻĀĶ¼ĖłŹ¾ø÷Ļī±ä»ÆµÄĪ“ÖŖŹżÖŠ¾łŗ¬ÓŠÄĘŌŖĖŲ£¬EĪŖµ»ĘÉ«·ŪÄ©£¬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ

¢ŁŠ“³öA”śBµÄ»Æѧ·½³ĢŹ½£¬±ź³öµē×Ó×ŖŅʵķ½ĻņŗĶŹżÄ棻
____________________________________________________________
¢ŚŌŚB”śCµÄ±ä»ÆÖŠĖłµĆCµÄČÜŅŗĶłĶł²»“棬ĘäÖŠµÄŌÓÖŹ£Ø²»°üĄØĖ®£©æÉÄÜŹĒ £¬Ö÷ŅŖŌŅņŹĒ £»»¹æÉÄÜŹĒ £¬Ö÷ŅŖŌŅņŹĒ ”£
(1) ¢Ł2Al + 2H2O + 2OH- = AlO2- + 3H2 ”ü»ņ 2Al + 6H+ == 2Al3+ + 3H2”ü
¢Ś 2FeCl2
+ Cl2 ==3 FeCl3
¢Ū 2Cu + O2  2CuO
2CuO
(2) £»æÉÄÜŹĒNaOH £¬ŅņCO2 ĮæÉŁ£¬NaOH¹żĮæ;æÉÄÜŹĒNaHCO3 £¬ŅņCO2 ¹żĮ棬Na2CO3×Ŗ»ÆĪŖNaHCO3
”¾½āĪö”æ£Ø1£©¢ŁĀĮÄÜČÜÓŚĒāŃõ»ÆÄĘČÜŅŗŗĶŃĪĖįÖŠ£¬¶ųĶ²»ÄÜ£¬¾Ż“ĖæÉŅŌ³żČ„ĀĮ£¬·½³ĢŹ½ĪŖ2Al + 2H2O + 2OH- = AlO2- + 3H2 ”ü»ņ 2Al + 6H+ == 2Al3+ + 3H2”ü”£
¢ŚĀČ»ÆŃĒĢś¾ßÓŠ»¹ŌŠŌ£¬Äܱ»ĀČĘųŃõ»ÆÉś³ÉĀČ»ÆĢś£¬ĖłŅŌ³żČ„ĀČ»ÆĢśÖŠµÄĀČ»ÆŃĒĢś£¬æÉŅŌĀČĘų£¬·½³ĢŹ½ĪŖ2FeCl2 + Cl2 ==3 FeCl3”£
¢ŪŃõĘųÄÜŗĶĶ·“Ó¦£¬µ«µŖĘų²»ÄÜ£¬ĖłŅŌŅŖ³żČ„µŖĘųÖŠµÄŃõĘų£¬æÉŅŌĶعż¼ÓČȵÄĶ£¬·½³ĢŹ½ĪŖ2Cu + O2  2CuO ”£
2CuO ӣ
£Ø2£©EĪŖµ»ĘÉ«·ŪÄ©£¬ĒŅŗ¬ÓŠÄĘŌŖĖŲ£¬ĖłŅŌEŹĒ¹żŃõ»ÆÄĘ”£ŌņAŹĒÄĘ£¬BŹĒĒāŃõ»ÆÄĘ£¬CŹĒĢ¼ĖįÄĘ£¬FŹĒĢ¼ĖįĒāÄĘ£¬DŹĒĀČ»ÆÄĘ”£
¢ŁÄĘŗĶĖ®µÄ·“Ó¦ÖŠ£¬ÄĘŹ§Č„µē×Ó£¬×÷»¹Ō¼Į£¬Ė®ŹĒŃõ»Æ¼ĮµĆµ½µē×Ó£¬·½³ĢŹ½ĪŖ”£
¢ŚCO2ŗĶĒāŃõ»ÆÄĘ·“Ó¦ÖŠ£¬Čē¹ūĒāŃõ»ÆÄĘ¹żĮæŌņÉś³ÉĪļĢ¼ĖįÄĘÖŠ»į»ģÓŠĒāŃõ»ÆÄĘ£»Čē¹ūCO2¹żĮ棬Ōņ²æ·ÖĢ¼ĖįÄĘÄÜŗĶCO2·“Ӧɜ³ÉĢ¼ĖįĒāÄĘ£¬ĖłŅŌŌÓÖŹæÉÄÜŹĒĢ¼ĖįĒāÄĘ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(12·Ö) øł¾ŻŅŖĒóĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©·ÖĪöĻĀĮŠĪļÖŹµÄĪļĄķŠŌÖŹ£¬ÅŠ¶ĻĘ侧ĢåĄąŠĶ£ŗ
A”¢¹ĢĢ¬Ź±Äܵ¼µē£¬ÄÜČÜÓŚŃĪĖį£»B”¢ÄÜČÜÓŚCS2£¬²»ČÜÓŚĖ®£»C”¢¹ĢĢ¬”¢ŅŗĢ¬Ź±¾ł²»µ¼µē£¬ČŪµć3500”ę
AӢ BӢ CӢ
£Ø2£©Öø³öÅäŗĻĪļK3[Co(CN)6]ÖŠµÄÖŠŠÄĄė×Ó”¢ÅäĢå¼°ĘäÅäĪ»Źż£ŗ_________”¢__________”¢_________”£
(3)ŌŚH2”¢SiC”¢CO2”¢HFÖŠ£¬Óɼ«ŠŌ¼ü×é³ÉµÄ·Ē¼«ŠŌ·Ö×ÓŹĒ £¬ÓÉ·Ē¼«ŠŌ¼üŠĪ³ÉµÄ·Ē¼«ŠŌ·Ö×ÓŹĒ £¬ÄÜŠĪ³É·Ö×Ó¾§ĢåµÄ»ÆŗĻĪļŹĒ £¬ŗ¬ÓŠĒā¼üµÄ¾§ĢåµÄ»ÆѧŹ½ £¬ŹōÓŚŌ×Ó¾§ĢåµÄŹĒ £¬ĖÄÖÖĪļÖŹČŪµćÓÉøßµ½µĶµÄĖ³ŠņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”Į¬ÖŻŹŠĮ¬ÖŻÖŠŃ§ø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
(7·Ö)øł¾ŻŅŖĒó»Ų“šĻĀĮŠĪŹĢā
(1)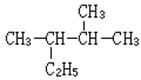 ________________________________ £ØŠ“Ćū³Ę£©
________________________________ £ØŠ“Ćū³Ę£©
£Ø2£©2£¼×»ł£2£ĪģĻ© £ØŠ“½į¹¹¼ņŹ½£©
£Ø3£©¼üĻߏ½ ±ķŹ¾µÄ·Ö×ÓŹ½ ”£
±ķŹ¾µÄ·Ö×ÓŹ½ ”£
(4)°“¹ŁÄÜĶŵIJ»Ķ¬£¬¶ŌÓŠ»śĪļ½ųŠŠ·ÖĄą£¬ĒėÖø³öĻĀĮŠÓŠ»śĪļµÄÖÖĄą£¬ĢīŌŚŗįĻßÉĻ”£
CH3CH2CH2Br __________£» ___________£»
___________£»  ________________£»
________________£» ________________£»
________________£»
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ°²»ÕŹ”Įł°²ČżÖŠøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧA¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
(12·Ö)øł¾ŻŅŖĒó»Ų“šĻą¹ŲĪŹĢā£ŗ
(1) ³żČ„ĻĀĮŠĪļÖŹÖŠĖł»ģÓŠµÄÉŁĮæŌÓÖŹ(ĄØŗÅÄŚĪŖŌÓÖŹ)£¬Š“³öÓŠ¹ŲµÄ·“Ó¦·½³ĢŹ½”£
¢ŁĶ·Ū (ĀĮ·Ū) £»
¢ŚFeCl3ČÜŅŗ(FeCl2 ) £»
¢ŪN2 (O2) ____________________________________________________”£
(2)ĻĀĶ¼ĖłŹ¾ø÷Ļī±ä»ÆµÄĪ“ÖŖŹżÖŠ¾łŗ¬ÓŠÄĘŌŖĖŲ£¬EĪŖµ»ĘÉ«·ŪÄ©£¬¾Ż“Ė»Ų“šĻĀĮŠĪŹĢā£ŗ 
¢ŁŠ“³öA”śBµÄ»Æѧ·½³ĢŹ½£¬±ź³öµē×Ó×ŖŅʵķ½ĻņŗĶŹżÄ棻
____________________________________________________________
¢ŚŌŚB”śCµÄ±ä»ÆÖŠĖłµĆCµÄČÜŅŗĶłĶł²»“棬ĘäÖŠµÄŌÓÖŹ£Ø²»°üĄØĖ®£©æÉÄÜŹĒ £¬Ö÷ŅŖŌŅņŹĒ £»»¹æÉÄÜŹĒ £¬Ö÷ŅŖŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011--2012ѧğĖÄ“ØŹ”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
(12·Ö) øł¾ŻŅŖĒóĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©·ÖĪöĻĀĮŠĪļÖŹµÄĪļĄķŠŌÖŹ£¬ÅŠ¶ĻĘ侧ĢåĄąŠĶ£ŗ
A”¢¹ĢĢ¬Ź±Äܵ¼µē£¬ÄÜČÜÓŚŃĪĖį£»B”¢ÄÜČÜÓŚCS2£¬²»ČÜÓŚĖ®£»C”¢¹ĢĢ¬”¢ŅŗĢ¬Ź±¾ł²»µ¼µē£¬ČŪµć3500”ę
AӢ BӢ CӢ
£Ø2£©Öø³öÅäŗĻĪļK3[Co(CN)6]ÖŠµÄÖŠŠÄĄė×Ó”¢ÅäĢå¼°ĘäÅäĪ»Źż£ŗ_________”¢__________”¢_________”£
(3)ŌŚH2”¢SiC”¢CO2”¢HFÖŠ£¬Óɼ«ŠŌ¼ü×é³ÉµÄ·Ē¼«ŠŌ·Ö×ÓŹĒ £¬ÓÉ·Ē¼«ŠŌ¼üŠĪ³ÉµÄ·Ē¼«ŠŌ·Ö×ÓŹĒ £¬ÄÜŠĪ³É·Ö×Ó¾§ĢåµÄ»ÆŗĻĪļŹĒ £¬ŗ¬ÓŠĒā¼üµÄ¾§ĢåµÄ»ÆѧŹ½ £¬ŹōÓŚŌ×Ó¾§ĢåµÄŹĒ £¬ĖÄÖÖĪļÖŹČŪµćÓÉøßµ½µĶµÄĖ³ŠņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com