
 Fe2O3+3H2O����֪���պ����ΪFe2O3��������Ԫ���غ㣬��֪��Ԫ�ص������������Ʒ����Ԫ�ص�����������
Fe2O3+3H2O����֪���պ����ΪFe2O3��������Ԫ���غ㣬��֪��Ԫ�ص������������Ʒ����Ԫ�ص����������� Fe2O3+3H2O����֪���պ����ΪFe2O3��Fe2O3������Ϊ��W2-W1������Ԫ�ص�����Ϊ��
Fe2O3+3H2O����֪���պ����ΪFe2O3��Fe2O3������Ϊ��W2-W1������Ԫ�ص�����Ϊ�� ��W2-W1������Ʒ������������
��W2-W1������Ʒ������������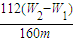 ×100%=
×100%= %��
%��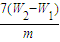 %��
%�� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 700��W2-W1�� |
| a |
| 700��W2-W1�� |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013�갲�����������ѧ������ѧ��ʵ�����¿���ѧ�Ծ����������� ���ͣ�ʵ����
(13��)ij�ֺ�������FeCl2���ʵ�FeCl3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ��������²�����У�
��ȷ����m g��Ʒ��2~3g����
������Ʒ�м���10mL 5mol/L�����ᣬ�ټ�������ˮ�����Ƴ�250mL��Һ��
����ȡ25mL����������õ���Һ������3mL��ˮ������ʹ֮��ȫ��Ӧ��
�ܳ���Ѹ�ټ���Ũ��Ϊ10%�İ�ˮ����������ֽ��裬ʹ֮��ȫ������
�ݹ��ˣ�������ϴ�ӡ����ա���ȴ�������������������ء�
���������������ش�
��1��������ǰ��������ƽ��ָ��ƫ��������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������ ��
A����mg�� B����mg�� C��ǡ��Ϊmg
��2���ܽ���ƷʱҪ�������ᣬԭ���� ��
��3������250mL��Һʱ������250mL������ƿ���ձ��⣬�����õ��IJ��������� ��
��4��������ˮʱ������Ӧ�����ӷ���ʽ�� ��
��5������������ΪW1 g�����������պ�������������W2 g������Ʒ����Ԫ�ص����������� ��
��6����������250mL��Һʱ�����õ�����ƿû��ϴ�ɾ�����������������ʱ�����ջ�ʹ��Ԫ�صIJⶨ�������ƫ�ߡ�����ƫ�͡����䡱����
��NaCl ��Fe2(SO4)3 ��Mg(NO3)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ����һ�и�����ѧ�ڵ�һ���¿���ѧ���� ���ͣ�ʵ����
(12��)ij�ֺ�������FeCl2���ʵ�FeCl3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ��������²�����У�
��ȷ����m g��Ʒ��2~3g����
������Ʒ�м���10mL 5mol/L�����ᣬ�ټ�������ˮ�����Ƴ�250mL��Һ��
����ȡ25mL����������õ���Һ������3mL��ˮ������ʹ֮��ȫ��Ӧ��
�ܳ���Ѹ�ټ���Ũ��Ϊ10%�İ�ˮ����������ֽ��裬ʹ֮��ȫ������
�ݹ��ˣ�������ϴ�ӡ����ա���ȴ�������������������ء�
���������������ش�
��1��������ǰ��������ƽ��ָ��ƫ��������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������ ��
A����mg�� B����mg�� C��ǡ��Ϊmg
��2���ܽ���ƷʱҪ�������ᣬԭ���� ��
��3������250mL��Һʱ������250mL������ƿ���ձ��⣬�����õ��IJ��������� ��
��4��������ˮʱ������Ӧ�����ӷ���ʽ�� ��
��5������������ΪW1 g�����������պ�������������W2 g������Ʒ����Ԫ�ص����������� ��
��6����������250mL��Һʱ�����õ�����ƿû��ϴ�ɾ�����������������ʱ�����ջ�ʹ��Ԫ�صIJⶨ�������ƫ�ߡ�����ƫ�͡����䡱����
��NaCl ��Fe2(SO4)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲�����������ѧ������ѧ��ʵ�����¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
(13��)ij�ֺ�������FeCl2���ʵ�FeCl3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ��������²�����У�
��ȷ����m g��Ʒ��2~3g����
������Ʒ�м���10mL 5mol/L�����ᣬ�ټ�������ˮ�����Ƴ�250mL��Һ��
����ȡ25mL����������õ���Һ������3mL��ˮ������ʹ֮��ȫ��Ӧ��
�ܳ���Ѹ�ټ���Ũ��Ϊ10%�İ�ˮ����������ֽ��裬ʹ֮��ȫ������
�ݹ��ˣ�������ϴ�ӡ����ա���ȴ�������������������ء�
���������������ش�
��1��������ǰ��������ƽ��ָ��ƫ��������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������ ��
A����mg�� B����mg�� C��ǡ��Ϊmg
��2���ܽ���ƷʱҪ�������ᣬԭ���� ��
��3������250mL��Һʱ������250mL������ƿ���ձ��⣬�����õ��IJ��������� ��
��4��������ˮʱ������Ӧ�����ӷ���ʽ�� ��
��5������������ΪW1 g�����������պ�������������W2 g������Ʒ����Ԫ�ص����������� ��
��6����������250mL��Һʱ�����õ�����ƿû��ϴ�ɾ�����������������ʱ�����ջ�ʹ��Ԫ�صIJⶨ�������ƫ�ߡ�����ƫ�͡����䡱����
��NaCl ��Fe2(SO4)3 ��Mg(NO3)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�ֺ�������FeCl2���ʵ�FeCl3��Ʒ����Ҫ�ⶨ������Ԫ�صĺ�����ʵ��������²�����У�
��ȷ����m g��Ʒ��2~3g����
������Ʒ�м���10mL 5mol/L�����ᣬ�ټ�������ˮ�����Ƴ�250mL��Һ��
����ȡ25mL����������õ���Һ������3mL��ˮ������ʹ֮��ȫ��Ӧ��
�ܳ���Ѹ�ټ���Ũ��Ϊ10%�İ�ˮ����������ֽ��裬ʹ֮��ȫ������
�� ���ˣ�������ϴ�ӡ����ա���ȴ�������������������ء�
���������������ش�
��1��������ǰ��������ƽ��ָ��ƫ��������������ʱ��ָ��պ��ڱ�ߵ��м䣬��������Ʒ������ ��
A����mg�� B����mg�� C��ǡ��Ϊmg
��2���ܽ���ƷʱҪ�������ᣬԭ���� ��
��3������250mL��Һʱ������250mL������ƿ���ձ��⣬�����õ��IJ������� �� ��
��4��������ˮʱ������Ӧ�����ӷ���ʽ�� ��
��5������������ΪW1 g�����������պ�������������W2 g������Ʒ����Ԫ�ص����������� ��
��6����������250mL��Һʱ�����õ�����ƿû��ϴ�ɾ�����������������ʱ�����ջ�ʹ��Ԫ�صIJⶨ�������ƫ�ߡ�����ƫ�͡����䡱����
��NaCl ��Fe2��SO4��3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com