
���л�ѧʵ����ʵ������Ͳ���ȷ����(����)
A�����з�̪��NaHCO3��Һ��dz��ɫ���Ⱥ��ɫ�������ΪNaHCO3�ֽ�������Na2CO3
B���Ʊ�����ú���У�����Ϊú�Ͳ����Ʒ�����Ӧ���Ʊ�ú���ܶȴ�ú�Ϳ���ʹ�Ƹ���������ˮ����
C���ýྻ�IJ����������Na2O2����֬��������֬��ȼ�գ�˵��CO2��H2O��Na2O2�ķ�Ӧ�Ƿ��ȷ�Ӧ
D���Ƴ��ڱ�¶�ڿ����еIJ�����Na2CO3��ԭ���������������ɵ�Na2O��ˮ�Ͷ�����̼��Ӧ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ����ij����������ʯ(��Fe2O3����)Ϊԭ��ұ�����Ĺ����������£�
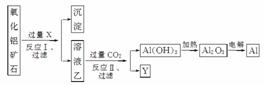
�����������е��ж���ȷ����(����)
A���Լ�X����Ϊ��ˮ�������к������Ļ�����
B��CO2������H2SO4��Һ��ϡ�������
C����Ӧ���еķ�ӦΪCO2��AlO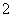 ��2H2O===Al(OH)3����HCO
��2H2O===Al(OH)3����HCO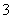
D����ҵ�ϻ��ɲ���Fe��ԭAl2O3�ķ�����Al���ɱ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ����С��Ϊ�˼���̼���ƺ�̼���������ְ�ɫ���壬�ò�ͬ�ķ�����������ʵ�飬����ͼ����ʾ��
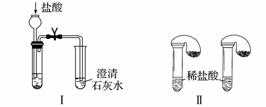
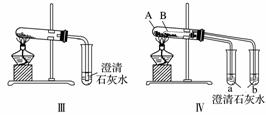
(1)ֻ����ͼ����ʾʵ�飬�ܹ��ﵽʵ��Ŀ�ĵ���________________________________________________________________________
(��װ�����)��
(2)ͼ����ʾʵ����ܼ������������ʣ��䷴Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
��ʵ�����ȣ�ʵ������ŵ���________(��ѡ�����)��
A�����Ȣ���
B�����Ȣ�ȫ
C�����Ȣ�������
D��������������һ��װ��ͬʱ���������Ա�ʵ�飬������
(3)����ʵ�����֤̼���ƺ�̼�����Ƶ��ȶ��ԣ����Թ�B��װ��Ĺ��������________________________________________________________________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
þ�Ǻ�ˮ�к����϶�Ľ�����þ��þ�Ͻ���þ�Ļ������ڿ�ѧ�о���ҵ��������;�dz��㷺��
(1)��ҵ�Ͽ��õ�����ڵ���ˮ�Ȼ�þ���þ�������Ȼ�þ��ˮ�ǹؼ�����֮һ��һ������������Ȼ�þ������ˮ�ķ����ǣ��Ƚ�MgCl2·6H2Oת��ΪMgCl2·NH4Cl·nNH3(�þ����)��Ȼ����700 ���Ѱ��õ���ˮ�Ȼ�þ���Ѱ���Ӧ�Ļ�ѧ����ʽΪ____________________��
��������Ȼ�þ�������ĵ缫��ӦʽΪ_______________________��
(2)�������Mg(AlH4)2��110��200 ��ķ�ӦΪMg(AlH4)2===MgH2��2Al��3H2����ÿ����27 g Alת�Ƶ��ӵ����ʵ���Ϊ____________��
(3)��ҵ����MgC2O4·2H2O�ȷֽ��Ƴ�ϸMgO�����ȷֽ���������ͼ��
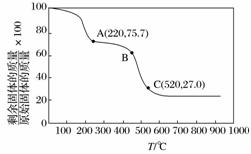
ͼ�и�������������B��C������Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���Ӧ�У������ﲻ�淴Ӧ������Ӧ��������仯���仯����(����)
A��Na��O2 B��NaOH��CO2
C��NaHCO3��NaOH D��Na2CO3��HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ���ۺ����ÿ����Ʊ�����þ�����������£�

(1)��ˮ��þҪ�õ���̲�ϵı��ǣ�����������________________________________�����Ǿ������йر仯�Ļ�ѧ����ʽ��____________________��
(2)д����MgCl2�õ�����þ�ķ�Ӧ����ʽ________________________________________________________________________
________________________________________________________________________��
(3)Mg(OH)2�����л���Ca(OH)2Ӧ������ȥ��(д��ʵ�鲽��)________________________________________________________________________��
(4)�Ӿ���Ч��Ƕȿ����û������ij�ַӦѡ����________________________________________________________________________
________________________________________________________________________��
(5)ʵ�����н������Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ����������ֱ�˵����������������õ���������Ŀ�ġ�
�ܽ�ʱ��________________________________________________________________________��
����ʱ��________________________________________________________________________��
����ʱ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A����ΪSO2����Ư���ԣ���������ʹƷ����Һ����ˮ������KMnO4��Һ��ʯ����Һ��ɫ
B����ʹƷ����Һ��ɫ�����ʲ�һ����SO2
C��SO2��Ư�ۡ�����̿��Na2O2����ʹ��īˮ��ɫ����ԭ����ͬ
D�������ʵ�����SO2��Cl2��Ϻ�ͨ��װ��ʪ�����ɫ�����ļ���ƿ�У�Ư��Ч������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������(Na2S2O3)�����������Լ�������Ļ�ԭ���������ȡ������ֽ⡣��ҵ�Ͽ��÷�Ӧ��2Na2S��Na2CO3��4SO2===3Na2S2O3��CO2 �Ƶá�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ���ش��������⣺

(1)b�з�Ӧ�����ӷ���ʽΪ__________________________________��
c���Լ�Ϊ____________��
(2)��Ӧ��ʼ��c�����л��Dz��������ֱ���塣�˻�������____________��
(3)d�е��Լ�Ϊ______________��
(4)ʵ����Ҫ����SO2�������ʣ����Բ�ȡ�Ĵ�ʩ��
________________________________________________________________________
___________________________________________________(�����)��
(5)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ��SO2���ܹ�����ԭ����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��W��M����Ԫ��λ�����ڱ���ǰ�����ڣ�ԭ������������������XԪ������������ḻ��Ԫ�أ�YԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ��WԪ��ԭ���������������ڲ��������3����M����δ�ɶԵ���������Ԫ�ء���ش��������⡣
(1)Y��Z��W�ĵ縺����С�����˳��Ϊ______________��
(2)Mԭ�ӵ���Χ�����Ų�ʽΪ_____��
(3) X2W2������Wԭ�ӹ�����ӻ�����Ϊ________��
(4)Z��W���γɵ�һ��ZW3-�����ӣ����以Ϊ�ȵ������������Ϊ___(��дһ��)��
(5)MCl3����Z��W���⻯���γ���λ��Ϊ6����������Ӧ������������ʵ���֮��Ϊ2��1��������ȫ��λ����硣��������Ļ�ѧʽΪ_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com