
I. ��ͼ1Ϊ��![]() ��Һ����εμ�
��Һ����εμ�![]() ��Һ��������Һ
��Һ��������Һ![]() �ı仯���ߡ���ش�
�ı仯���ߡ���ش�
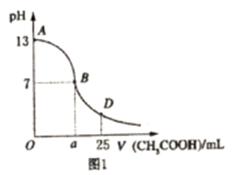
��1��B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱ![]() ��
��![]() ǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��__________��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�__________���䣨����ȷ�����ʲ��𣩡�
ǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��__________��ѡ��ǡ�����������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�__________���䣨����ȷ�����ʲ��𣩡�
��2�����ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ��________��ѡ����ĸ����
| ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� | |
| A | �� | �� | ��̪ | ���ף� |
| B | �� | �� | ���� | ���ף� |
| C | �� | �� | ʯ�� | ���ң� |
| D | �� | �� | ʯ�� | ���ң� |
��3��AB���䣬![]() ����
����![]() ��
��![]() ��С��ϵ��________��
��С��ϵ��________��
A. ![]() ����
����![]() B.
B. ![]() ��
��![]()
C. ![]() ����
����![]() D. �����������������
D. �����������������
��4����D��ʱ����Һ��![]() ________
________![]() ���>����<����=������
���>����<����=������
II. ![]() ��ʱ��ijϡ������Һ��
��ʱ��ijϡ������Һ��![]() ��
��![]() ����֪
����֪![]() ��
��
��5�����¶���ˮ�����ӻ�����![]() ����ֵΪ_________��
����ֵΪ_________��
��6�����¶��£�![]() �棩����
�棩����![]() ��ϡ
��ϡ![]() ��
��![]() ��
��![]() ��Һ��ϣ���Һ����仯���Բ��ƣ�����Һ��
��Һ��ϣ���Һ����仯���Բ��ƣ�����Һ��![]() =_________��
=_________��
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�ʹ�һ�и�������μ�⻯ѧ�Ծ����������� ���ͣ������
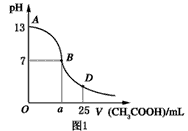
I����ͼ1Ϊ��25mL 0.1mol��L��1NaOH��Һ����εμ�0.2mol��L��1CH3COOH��Һ��������ҺpH�ı仯���ߡ���ش�
�� B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��________(ѡ��ǡ���)��������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�________����(����ȷ�����ʲ���)��
�ƹ��ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ��________(ѡ����ĸ)��
| | ��ƿ����Һ | �ζ�������Һ | ѡ��ָʾ�� | ѡ�õζ��� |
| A | �� | �� | ��̪ | (��) |
| B | �� | �� | ���� | (��) |
| C | �� | �� | ʯ�� | (��) |
| D | �� | �� | ��̪ | (��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��������μ�⻯ѧ�Ծ��������棩 ���ͣ������
I����ͼ1Ϊ��25mL 0.1mol��L��1NaOH��Һ����εμ�0.2mol��L��1CH3COOH��Һ��������ҺpH�ı仯���ߡ���ش�

�� B����Һ�����ԣ����˾ݴ���Ϊ����B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ�����ֿ����Ƿ���ȷ��________(ѡ��ǡ���)��������ȷ�������ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD�����ڣ�________����(����ȷ�����ʲ���)��
�ƹ��ڸõζ�ʵ�飬������ѡ����ѡ����ǡ����һ��________(ѡ����ĸ)��

��ƿ����Һ �ζ�������Һ ѡ��ָʾ�� ѡ�õζ���
A �� �� ��̪ (��)
B �� �� ���� (��)
C �� �� ʯ�� (��)
D �� �� ��̪ (��)
�� AB���䣬c(OH��)>c(H+)����c(OH��)��c(CH3COO��)��С��ϵ��________��
A��c(OH��)����c(CH3COO��) B��c(OH��)��c(CH3COO��)
C��c(OH��)����c(CH3COO��) D�������������������
����D��ʱ����Һ��c(CH3COO��)��c(CH3COOH)________2c(Na+)(�>����<������)��
��t��ʱ��ijϡ������Һ��c(H+) �� 10��a mol��L��1��c(OH��) �� 10��b mol��L��1����֪a��b��13:
�ɸ��¶���ˮ�����ӻ�����Kw����ֵΪ________��
�ʸ��¶���(t��)����100mL 0.1mol��L��1��ϡH2SO4��100mL 0.4mol��L��1��NaOH��Һ���(��Һ����仯���Բ���)����Һ��pH��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ĩ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ĩ�� ���ͣ������


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com