
�����������ɷ������·�Ӧ��P4��5O2 == P4O10����֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��P��P akJ��mol��1��P��O b kJ��mol��1��P��O c kJ��mol��1��O��O d kJ��mol��1������ͼʾ�ķ��� �ṹ���й����ݹ���÷�Ӧ�Ħ�H��������ȷ����( )
�ṹ���й����ݹ���÷�Ӧ�Ħ�H��������ȷ����( )

A��(6a��5d��4c��12b)kJ��mol��1 B��(4c��12b��6a��5d)kJ��mol��1
C��(4c��12b��4a��5d)kJ��mol��1 D��(4a��5d��4c��12b)kJ��mol��1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ɳ�и�һ�ϵ�һ��˫������ѧ���������棩 ���ͣ�ѡ����
����Ħ����˵������ȷ����( )
A��Ħ���DZ�ʾ���������ĵ�λ
B��Ħ���DZ�ʾ���ʶ��ٵĵ�λ
C��Ħ���Ǽȱ�ʾ�������������������������ܱ�ʾ���ʵ���������˫������ĵ�λ
D��Ħ���Ǿ���Ŀ�����Ӽ����壬��ʾ���ʵ����Ĺ��ʵ�λ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017���������ľ˹һ�и�һ��9.10������ѧ���������棩 ���ͣ�ѡ����
����˵������ȷ���ǣ�NA��ʾ����٤��������ֵ���� ��
A��28gN2�����е�ԭ����ΪNA
B���ڳ��³�ѹ�£�11.2LCl2���еķ�����Ϊ0.5NA
C��22.4LCH4��������NA��������ӵ��������
D����״���£�22.4LCH4��O2�Ļ�����������еķ�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���9���¿���ѧ���������棩 ���ͣ�ѡ����
�е��������pH��Ca��OH��2��KOH��NH3•H2O���ּ���Һ���μӵ�Ũ�ȵ����Ὣ����ǡ���кͣ���ȥ�������ֱ�ΪV1��V2��V3�������ߵĴ�С��ϵ��ȷ���ǣ� ��
A��V3��V2��V1 B��V3=V2=V1 C��V3��V2=V1 D��V1=V2��V3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶���9���¿���ѧ���������棩 ���ͣ�ѡ����
Ϊ��̽����������Է�ӦaX��g��+bY��g�� cZ��g����Ӱ�죬��X��Y���ʵ�����Ϊa��b��ʼ��Ӧ��ͨ��ʵ��õ���ͬ�����´ﵽƽ��ʱZ�����ʵ���������ʵ��������ͼ��ʾ�������ж���ȷ���ǣ�
cZ��g����Ӱ�죬��X��Y���ʵ�����Ϊa��b��ʼ��Ӧ��ͨ��ʵ��õ���ͬ�����´ﵽƽ��ʱZ�����ʵ���������ʵ��������ͼ��ʾ�������ж���ȷ���ǣ�  ��
��

A����H��0��a+b��c B����H��0��a+b��c
C����H��0��a+b��c D����H��0��a+b��c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ������
ClO2������һ�ֳ��õ����������ҹ���2 000��������ClO2��������������ˮ����������
��1������ˮʱ��ClO2���ɽ�ˮ�е�Fe2����Mn2����ת����Fe(OH)3��MnO2���������ȥ������������У�Fe2����Mn2���Ļ��ϼ����ߣ�˵��ClO2����__________�ԡ�
��2����ҵ�Ͽ���ͨ�����з�����ȡClO2������ɸû�ѧ��Ӧ����ʽ��2KClO3��SO2=2ClO2��__________��
��3��ʹCl2��H2O(g)ͨ�����ȵ�̿�㣬����HCl��CO2���Ƿ��ȷ�Ӧ����1molCl2���뷴Ӧʱ�ͷ�145kJ��������д�������Ӧ���Ȼ�ѧ����ʽ��___________________��
��4������ˮ����ClO2�������ˮ�У�Ҫ��ClO2��Ũ����0��1��0��8mg��L��1֮�䡣���������Լ��ˮ��ClO2��Ũ�ȣ��������£�
��ȡһ�������ˮ��������һ�����ĵ⻯�أ���������������Һ�������ԣ������������Һ����Һ������
����һ������Na2S2O3��Һ��(��֪��2S2O ��I2=S4O
��I2=S4O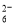 ��2I��)
��2I��)
���������ˮ��pH��1��3��
����ʱ����ͬpH������������������ͼ��ʾ��

��ش�
�ٲ������з�Ӧ�����ӷ���ʽ��______________________��
��ȷ����������ȫ��Ӧ��������___________________��
���ڲ���������У���Һ�ֳ���ɫ����Ӧ�����ӷ���ʽ��____________________��
����ˮ�������Ϊ1��0 L���ڲ�����ʱ������1��0��10��3 mol��L��1��Na2S2O3��Һ10 mL����ˮ����ClO2��Ũ����________mg��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ��9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���Ȼ�����S2C12���ǹ㷺������ҵ����������ӽṹ����ͼ��ʾ�������£�S2C12��һ�ֳȻ�ɫҺ�壬��ˮ��ˮ�⣬��������ʹƷ����ɫ�����壬��ѧ����ʽΪ��2S2C12+2H2O��SO2��+3S��+4HCl������˵���д������
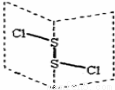
A��S2C12�ĽṹʽΪCl��S��S��Cl
B����Ӧ��SO2�ǻ�ԭ���S����������
C��S2C12Ϊ���м��Լ��ͷǼ��Լ��ķ���
D����Ӧ�У�����1molSO2��ת�Ƶ���Ϊ3mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ������
�����ǻ�����������Ҫ��Ʒ�����������������Ϊ����һ�����ҹ�ҵ��չˮƽ�ı�־�������������Ҫ��ӦΪ�� SO2(g)+ 1/2O2(g) SO3(g)
SO3(g)
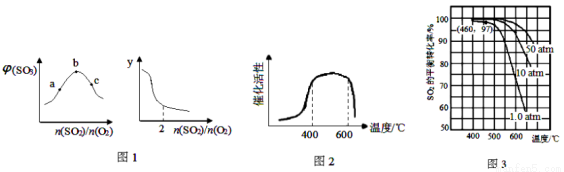
��1�����º����£�ƽ����ϵ��SO3���������[��(SO3)]��y��SO2��O2�����ʵ���֮��[n(SO2)/n(O2)]�Ĺ�ϵ��ͼ1����b��n(SO2)/n(O2)=_________��yΪ_________�����ţ���
A��ƽ�ⳣ�� B��SO3��ƽ����� C��O2��ƽ��ת���� D��SO2��ƽ��ת����
��2 Kp���Ը�����ƽ���ѹ����Ũ��ƽ�ⳣ��Kc�и������Ũ�ȵ�ƽ�ⳣ������400-650��ʱ��Kp���¶ȣ�TK���Ĺ�ϵΪ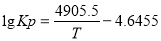 �����ڴ�������SO2ת��ΪSO3��Ӧ�ġ�H_________���>0����<0������
�����ڴ�������SO2ת��ΪSO3��Ӧ�ġ�H_________���>0����<0������
��3���ٸ÷�Ӧ�Ĵ���ΪV2O5�������Ӧ����Ϊ��
SO2+ V2O5 SO3+V2O4 K1
SO3+V2O4 K1
1/2 O2+V2O4  V2O5 K2
V2O5 K2
������ͬ�¶���2SO2(g)+ O2 (g)  2SO3 (g)��ƽ�ⳣ��K=________���Ժ�K1��K2�Ĵ���ʽ��ʾ����
2SO3 (g)��ƽ�ⳣ��K=________���Ժ�K1��K2�Ĵ���ʽ��ʾ����
��V2O5�ӿ췴Ӧ���ʵ�ԭ����___________������������¶ȵĹ�ϵ����ͼ2��
��4����7.0% SO2��11% O2��82% N2����ֵ��Ϊ�������������ʱ��SO2ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ3������ʽ����460�桢1.0 atm�£�SO2 (g)+l/2 O2 (g)  SO3 (g)��Kp=_________����֪��������ķ�ѹ=��ѹ��������������������
SO3 (g)��Kp=_________����֪��������ķ�ѹ=��ѹ��������������������
��5���ۺϵڣ�3������4����ͼ����Ϣ����ҵ���������˵��¶ȷ�ΧΪ____________��ѹǿͨ�����ó�ѹ��ԭ����_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��2H2(g) + O2(g) = 2H2O(l) ��H �� ��571.6 kJ��mol��1 ��CH4(g) + 2O2(g)= CO2(g) + 2H2O(l) ��H �� ��890 kJ��mol��1 ������H2��CH4�Ļ������112L����״������ʹ����ȫȼ������CO2��H2O(l)����ʵ���÷�Ӧ����3695kJ����ԭ���������H2��CH4�����ʵ���֮����
A��1��1 B��1��3 C��1��4 D��2��3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com