
 =O+RMgX��
=O+RMgX�� $\stackrel{H_{2}O}{��}$
$\stackrel{H_{2}O}{��}$ ������ϩ�ͱ�Ҫ������Ϊԭ�Ϻϳ�2-�����������ϳ�һ�ַ���ʽΪC6H8O4�ľ�����Ԫ��������J���ϳ���·��ͼ��G�������ֲ�ͬ��ѧ��������ԭ�ӣ�
������ϩ�ͱ�Ҫ������Ϊԭ�Ϻϳ�2-�����������ϳ�һ�ַ���ʽΪC6H8O4�ľ�����Ԫ��������J���ϳ���·��ͼ��G�������ֲ�ͬ��ѧ��������ԭ�ӣ�
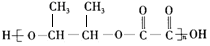 ��
�� ���� ��ϩ��HBr��Ӧ����AΪCH3CH2Br����ת����ϵ��֪BΪCH3CH2OH��DΪCH3CHO���������Ϣ��֪CΪCH3CH2MgBr��EΪCH3CH��CH2CH3��-OMgBr��
FΪCH3CHOHCH2CH3��G�������ֲ�ͬ��ѧ��������ԭ�ӣ���GΪCH3CH=CHCH3��HΪCH3CHBrCHBrCH3��IΪCH3CHOHCHOHCH3��J�ķ���ʽΪC6H8O4��������Ԫ������ӦΪ�Ҷ��ᷢ��������Ӧ���ɣ��Դ˽����⣮
��� �⣺��1�������Ϸ�����֪FΪCH3CHOHCH2CH3���ʴ�Ϊ��CH3CHOHCH2CH3��
��2��DΪCH3CHO����C��Ӧ����CH3CH��CH2CH3��-OMgBr��ӦΪ�ӳɷ�Ӧ��FΪCH3CHOHCH2CH3��������ȥ��Ӧ����G��
�ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
��3��A��B�ķ�ӦΪCH3CH2Br��ˮ�⣬����ʽΪCH3CH2Br+NaOH$��_{��}^{H_{2}O}$CH3CH2OH+NaBr��BΪCH3CH2OH��������������������ȩ������ʽΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
�ʴ�Ϊ��CH3CH2Br+NaOH$��_{��}^{H_{2}O}$CH3CH2OH+NaBr��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��4��IΪCH3CHOHCHOHCH3�����Ҷ��ᷢ�����۷�Ӧ����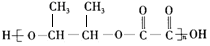 ���ʴ�Ϊ��
���ʴ�Ϊ��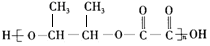 ��
��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬���ؿ���ѧ���ķ�����������Ŀ�ѶȽϴ���ע���������Ϣ��GΪ�������ͻ�ƿڣ����������Ƶķ��������ƶϣ�����ʱע����շ�Ӧ�����������ƶϹ����ŵı仯��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | M���ǵ��ص����� | |
| B�� | ��Һ�е���������N���ƶ� | |
| C�� | M���Ϸų���������ʹʪ��ĵ��۵⻯����ֽ���� | |
| D�� | ��M��N��������0.4mole-ʱ��N���ϻ�����6.4g�Ȼ�ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������SO2����ͨ��NaClO��Һ�У�SO2+2ClO-+H2O�TSO32-+2HClO | |
| B�� | Fe3O4�ܽ��ڹ�����ϡ�����У�Fe3O4+8H+�TFe2++2Fe3++4H2O | |
| C�� | NH4Al��SO4��2��Һ�������Ba��OH��2��Һ��ϣ�NH4++Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+Al��OH��3��+NH3•H2O | |
| D�� | ��NaAlO2��Һ��ͨ������CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ�п϶���SO42-��Cu2+��һ��û��Ba2+���������Ӳ���ȷ�� | |
| B�� | ��Һ�п϶���SO42-��Cu2+��������Na+��Cl- | |
| C�� | ��Һ�п϶���SO42-��Cu2+��Cl-��������Na+ | |
| D�� | ��Һ�����ͬʱ��Na+��Cl-����C��Na+����C��Cl-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ѡ���Լ� | ʵ������ | |
| ��һ�ַ��� | C | �л�����ɫ |
| �ڶ��ַ��� | D | ��Һ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ȷ������C�ijɷ� | |
| B�� | ��ȷ��ԭ��Һ���Ƿ���Al3+��Cl- | |
| C�� | ԭ��Һ�д��ڵ�����ΪNH4+��Fe2+��Cl-��SO42- | |
| D�� | ��ҺX�д������ڵ���������NH4+��Fe2+��Ba2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Һ������������̼��Ӧ��C6H5O-+CO2+H2O=C6H5OH+HCO3- | |
| B�� | ̼������������ʯ��ˮ��Ӧ��HCO3-+Ca2++OH-=CaCO3��+H2O | |
| C�� | ��������KHSO4��Һ�е���Ba��OH��2��Һʹ��ҺpH=7��SO42-+2H++Ba2++2OH-=BaSO4��+2H2O | |
| D�� | ��KAl��SO4��2��Һ�м�������������Һ��SO42-������ȫ��Al3++2SO42-+2Ba2++4OH-=2BaSO4��+AlO2-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪ�Զ��Ե缫���е���װ�ã�
��ͼΪ�Զ��Ե缫���е���װ�ã��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com