
��1���������з�Ӧ��ƽ������v��B��=__________,��ѧ����ʽ�м���ϵ��b��__________��
��2���������з�Ӧ�ﵽƽ������Ҫ��ʱ��t__________������ڡ���С�ڡ����ڡ���4 min��ԭ����_______________________________��
��3��T ��ʱ������һ���������������ͬ�ı������У�Ϊ��ʹ�ﵽƽ��ʱB��Ũ����ȻΪ0.8 mol��L-1����ʼʱ���������м���C��D�����ʵ����ֱ�Ϊ3 mol ��2 mol���������A��B�����ʵ����ֱ��ǣ�______________________��
��4����Ҫʹ�ס�����������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��__________��
A.�����¶Ȳ��䣬����������������2 L
B.��������������䣬ʹ�����������¶�
C.��������ѹǿ���¶ȶ����䣬����м���һ������A����
D.��������ѹǿ�����䣬����м���һ������B����
˼·��������1����Ӧǰ����������A��Ũ��Ϊ6 mol��L-1��4 min�������ڵķ�Ӧ�ﵽ��ѧƽ���A��Ũ�ȼ�����3.6 mol��L-1��B��Ũ�ȼ�����1.2 mol��L-1�����ݷ�Ӧ���ʵļ��㹫ʽ��ϻ�ѧ��Ӧ����ʽ�и����ʵĻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������v��B��=0.3 mol��L-1��min-1��b��1����2����������������ڼģ�Ũ��С����ƽ���ʱ�䳤����3�����ݵ�Чƽ����ɣ��ں��º����£�ת��Ϊͬһ��Ӧ��������ʵ����ʵ�����ԭ����Ӧ��ȼ��ɡ�3 mol C��2 mol D��ȫת��������3 mol A��1 mol B������3 mol A��2 mol B����4��������������ԭ������ϵ�Чƽ����ɣ���֪A��B��C���С�
�𰸣���1��0.3 mol��L-1��min-1 1
��2������ ��������������ڼ��������������Ӧ��Ũ�ȼ�С����Ӧ���ʼ������ﵽƽ������Ҫ��ʱ���Ҫ��
��3��3 mol��2 mol
��4��ABC



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
. |
| v |
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
A.�������з�Ӧ��ƽ������![]() (B)=0.3 mol/(L��min)
(B)=0.3 mol/(L��min)
B.��ѧ����ʽ�м�����b=1
C.�������з�Ӧ�ﵽƽ��ʱ����ʱ�����4 min
D.T ��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8 mol/L����ʼʱ����������м���3 mol C��2 mol D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
T��ʱ���мס��������ܱ������������������Ϊ1 L�������������Ϊ2 L���ֱ���ס����������м���6 mol A��3 mol B��������Ӧ���£�3A(g)��bB(g) 3C(g)��2D(g)����H<0; 4 minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬A��Ũ��Ϊ2.4 mol/L��B��Ũ��Ϊ1.8 mol/L; t minʱ�������ڵķ�Ӧ��ƽ�⣬B��Ũ��Ϊ0.8 mol/L.���������Ϣ�ش��������⣺
(1)�������з�Ӧ��ƽ������v(B)��________����ѧ����ʽ�м�����b��________.
(2)�������з�Ӧ�ﵽƽ��ʱ����ʱ��t________4min(����ڡ�����С�ڡ����ڡ�)��ԭ����______________________________��
(3)T��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8mol/L����ʼʱ����������м���C��D�����ʵ����ֱ�Ϊ3 mol��2 mol���������A��B�����ʵ����ֱ���________��________.
(4)��Ҫʹ�ס���������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��________��
A�������¶Ȳ��䣬����������������2 L
B����������������䣬ʹ�����������¶�
C����������ѹǿ���¶ȶ����䣬����м���һ������A����
D����������ѹǿ���¶ȶ����䣬����м���һ������B����
(5)д��ƽ�ⳣ������ʽK��________����������T��ʱ�Ļ�ѧƽ�ⳣ��K��________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ���ݶ��и߶��ڶ�ѧ�����п��Ի�ѧ���� ���ͣ������
��6�֣�T��ʱ���мס��������ܱ������������������Ϊ1L�������������Ϊ2L���ֱ���ס����������м���6mol A��3mol B��������Ӧ���£�3A��g����b B��g�� 3C��g����2D��g����
3C��g����2D��g����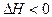 ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��1���������з�Ӧ��ƽ������v��B����_________����ѧ����ʽ�м�����b��________��
��2���������з�Ӧ�ﵽƽ������ʱ��t_________4min������ڡ�����С�ڡ����ڡ�����ԭ����___________________________________________________________��
��3��T��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8mol/L����ʼʱ����������м���C��D�����ʵ����ֱ�Ϊ3mol��2mol���������A��B�����ʵ����ֱ���___________��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ�߶��ڶ�ѧ�����п��Ի�ѧ���� ���ͣ������
��6�֣�T��ʱ���мס��������ܱ������������������Ϊ1L�������������Ϊ2L���ֱ���ס����������м���6mol A��3mol
B��������Ӧ���£�3A��g����b B��g�� 3C��g����2D��g����
3C��g����2D��g���� ��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��4min��������ڵķ�Ӧ�ﵽƽ�⣬A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L��t min���������ڵķ�Ӧ�ﵽƽ�⣬B��Ũ��Ϊ0.8mol/L�������������Ϣ�ش��������⣺
��1���������з�Ӧ��ƽ������v��B����_________����ѧ����ʽ�м�����b��________��
��2���������з�Ӧ�ﵽƽ������ʱ��t_________4min������ڡ�����С�ڡ����ڡ�����ԭ����___________________________________________________________��
��3��T��ʱ������һ�����������ͬ�ı������У�Ϊ�˴ﵽƽ��ʱB��Ũ����ȻΪ0.8mol/L����ʼʱ����������м���C��D�����ʵ����ֱ�Ϊ3mol��2mol���������A��B�����ʵ����ֱ���___________��___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com