

��ش��������⣺
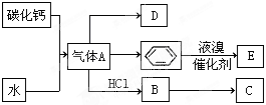
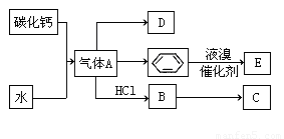
(1)����B�ĵ���ʽΪ____________�������������Ļ�ѧ����____________��
(2)����A�Ļ�ѧʽΪ____________�����зǽ���Ԫ�صĻ�ѧ��Ϊ____________�ۡ�
(3)��Ӧ�ܵ����ӷ���ʽΪ________________����Ӧ�ݵĻ�ѧ����ʽΪ_________________��
(4)��ͭ���缫���H��Һ�������ĵ缫��ӦʽΪ__________________________________��
(5)��֪ÿ����
(1) ![]() ���Ӽ������ۼ�(��Ǽ��Լ�)
���Ӽ������ۼ�(��Ǽ��Լ�)
(2)FeS2 -1
(3)Fe2O3+6H+![]() 2Fe3++3H2O
2Fe3++3H2O
Fe2(SO4)3+6NaOH![]() 2Fe(OH)3��+3Na2SO4
2Fe(OH)3��+3Na2SO4
(4)Cu-2e-![]() Cu2+
Cu2+
(5)FeS2(s)+11/4O2(g)![]() 1/2Fe2O3(s)+2SO2(g)����H=-853 kJ/mol
1/2Fe2O3(s)+2SO2(g)����H=-853 kJ/mol
������X������Ϊ��ɫ��ζ��Һ�壬֤��X��H2O����ɫ����BΪNa2O2��C��D�ֱ�ΪNaOH��O2���٢��ǻ�ѧ��ҵ�����е���Ҫ��Ӧ��JΪ���ɫ����Fe(OH)3������֪A��E��F�ֱ�ΪFeS2��SO2��Fe2O3����GΪSO3��HΪH2SO4��IΪFe2(SO4)3.
(4)��ͭ���缫���H2SO4ʱ��������ͭ�ŵ�����ܽ⣬Cu-2e-![]() Cu2+.
Cu2+.



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013��㶫��ɽ��ɽһ�и߶���ѧ�ڵ�һ�ζο���ѧ�Ծ����������� ���ͣ��ƶ���
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
��1��д��A�ĵ���ʽ ��D�����ʽ ��
��2��д��̼������ˮ��Ӧ��ȡA�Ļ�ѧ����ʽ ������Һ�巴Ӧ����E�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
B��C�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
a����ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
b��D��F�Ļ�ѧ����ʽ ���䷴Ӧ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ�߶���ѧ�ڵ�һ�ζο����ƻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
��1��д��A�ĵ���ʽ ��D�����ʽ ��
��2��д��̼������ˮ��Ӧ��ȡA�Ļ�ѧ����ʽ ������Һ�巴Ӧ����E�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��B��C�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
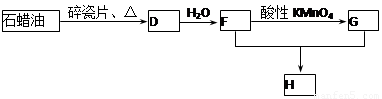
a����ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
b��D��F�Ļ�ѧ����ʽ ���䷴Ӧ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫��ɽ��ɽһ�и߶���ѧ�ڵ�һ�ζο���ѧ�Ծ��������棩 ���ͣ��ƶ���
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
��1��д��A�ĵ���ʽ ��D�����ʽ ��
��2��д��̼������ˮ��Ӧ��ȡA�Ļ�ѧ����ʽ ������Һ�巴Ӧ����E�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
B��C�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
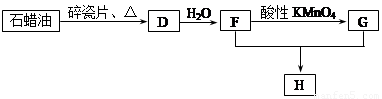
a����ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
b��D��F�Ļ�ѧ����ʽ ���䷴Ӧ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C��һ�ֺϳ���֬�������Ʊ����Ϻͺϳ���ά��D��һ��ֲ���������ڼ����������Դ����ʵ���������»�ѧ��Ӧ��ͼ��գ�
 |
��1��д��A�ĵ���ʽ ��D�����ʽ ��
��2��д��̼������ˮ��Ӧ��ȡA�Ļ�ѧ����ʽ ������Һ�巴Ӧ����E�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
B��C�Ļ�ѧ����ʽ ���䷴Ӧ����Ϊ ��
 ��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
��3��D��������ʯ������ȡ��D��һ�������´�������ת����ϵ��ʯ���ͺ�17��̼ԭ�����ϵ�Һ̬���������ַ�Ӧ���������ﱻʡ�ԣ���G��һ���������ʣ�H�Ǿ��й�����ζ�����������
a����ҵ�ϣ���ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����� ��
b��D��F�Ļ�ѧ����ʽ ���䷴Ӧ������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com