

| A������Һ�����Ӽ����㣺c(Na+)>c(CH3COO��)>c(OH��)>c(H+)������Һ�����ʿ���ΪCH3COONa��NaOH |
| B������Һ�����Ӽ�����c(CH3COO��)>c(Na+)>c(H+)>c (OH��)������Һ������һ��ֻ��CH3COONa |
| C������Һ��c(Na+)=c(CH3COO��)�������Һһ�������� |
| D������Һ��c(CH3COOH)>c(Na+)������Һһ�������� |
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���ܵ���� | AgCl | AgI | AgOH | Ag2S | PbI2 | Pb(OH)2 | PbS |
| Ksp | 1.8��10-10 | 8.3��10-17 | 5.6��10-18 | 6.3��10-50 | 7.1��10-9 | 1.2��10-15 | 3.4��10-28 |

| A��NaOH | B��Na2S | C��KI | D��Ca(OH)2 |
 ��2���������ʯ�Ҵ���������ˮ��ʹ��Һ��pH��8.0��������ķ�ˮ��c(Pb2+)�� ��
��2���������ʯ�Ҵ���������ˮ��ʹ��Һ��pH��8.0��������ķ�ˮ��c(Pb2+)�� �� ��3�������ʳ�δ�����ֻ�������ӵķ�ˮ����ô�����ķ�ˮ��NaCl����������Ϊ0.117%��������Ҫ���ŷű�Ϊc(Ag+)����1.0��10-18mol��L-1����ù���������ķ�ˮ�Ƿ�����ŷű� ��
��3�������ʳ�δ�����ֻ�������ӵķ�ˮ����ô�����ķ�ˮ��NaCl����������Ϊ0.117%��������Ҫ���ŷű�Ϊc(Ag+)����1.0��10-18mol��L-1����ù���������ķ�ˮ�Ƿ�����ŷű� �� ����ǡ��� ����д��������̡�
����ǡ��� ����д��������̡��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ǰ�ߴ��ں��� | B�����ߴ���ǰ�� | C��������� | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������Һ�����ᷴӦ HCOO-+H+====HCOOH |
B�����Ƶ�һ��ˮ��S2-+2H2O H2S+2OH- H2S+2OH- |
C�����ᱵ��Һ�����ᷴӦBa2++SO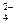 ====BaSO4�� ====BaSO4�� |
D������������Һ��̼����þ��ӦCa2++OH-+HCO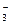 ====CaCO3��+H2O ====CaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ |
| B��һ���¶��£�IL 1mol��L�İ�ˮ��2L 0��5mol��L�İ�ˮ�У�n��NH4+��ǰ�߶� |
| C����ͬ�������ͬ���ʵ���Ũ�ȵ����ᡢ���ᣬϡ����ͬ��������Һ��pH������<���� |
| D��0��2mol��L��һԪ��HX��0��1mol��L��KOH��Һ��������������Һ�У�һ���У�a��H+��+c��K+��=c��OH-��+c��X-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ɫ��Һ��Cu2����K����MnO4����SO42�� |
| B�����ܽ�Al2O3����Һ��Na����Ca2����HCO3����NO3�� |
| C��c(OH��)��1��10��13mol��L��1����Һ��NH4����Al3����SO42����Cl�� |
| D����0.1 mol��L��1 NO3������Һ��H����Fe2����Mg2����Br�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ˮ�������ϸ��¶ȣ�ˮ�����ӻ����pH��С�������� |
| B����ˮ�������Ϻ���Һ�����ԣ���Һ������Ũ�ȴ�С��ϵһ�����ڣ�c��NH4+��>c��H+�� |
| C����ͬ�¶��£�pH=2�Ĵ�����Һ��pH=2��������c��H+��֮��Ϊ1��l |
| D����ʢ������Mg��OH��2�������Թ��м���NH4ClŨ��Һ�������������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com