
| A�������Ȼ�ѧ����ʽ��ȼ��1mol S�ų�������Ϊ297.23 kJ |
| B���γ�1 mol SO2�Ļ�ѧ�����ͷŵ����������ڶ��� 1 mol S ( s )�� 1mol O2 ( g )�Ļ�ѧ�������յ������� |
| C��S ( g ) + O2 ( g ) = SO2 ( g )��H =��Q kJ/mol��Q>297.23 |
| D��S ( g ) + O2 ( g ) = SO2 ( g )��H =��Q kJ/mol��Q<297.23 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
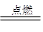 2H2O���Իش��������⡣
2H2O���Իش��������⡣�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
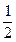 O2��g��====H2O��l����H����285kJ��mol��1
O2��g��====H2O��l����H����285kJ��mol��1 ����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1
����5O2��g��====3CO2��g����4H2O��l����H����2220.0kJ��mol��1 ��====H2O��g�� ��H����44.0kJ��mol��1��д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ_________________________________________��
��====H2O��g�� ��H����44.0kJ��mol��1��д������ȼ������CO2����̬ˮ���Ȼ�ѧ����ʽ_________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢ� | B���ڢ� | C���٢� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 O2(g)��H2O(l) ����H 3����285.8kJ/mol
O2(g)��H2O(l) ����H 3����285.8kJ/mol | A��488.3 kJ/mol | B����488.3 kJ/mol | C����244.15 kJ/mol | D��244.15 kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)
�ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol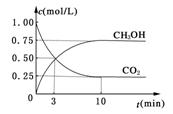
 n)��
n)�� ��H����1277 kJ��mol-1
��H����1277 kJ��mol-1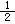 O2(g)��CO(g)�� ��H����110.5 kJ��mol-1
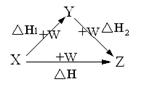
O2(g)��CO(g)�� ��H����110.5 kJ��mol-1 ���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��
���й���ѧԺ����Ӧ�û�ѧ�о����ڼ״�ȼ�ϵ�ؼ�����������ͻ�ƣ���װ�����Ժ�����ؼ�����ʽ��ѡ��״�ȼ�ϵ�صĹ���ԭ������ͼ��ʾ��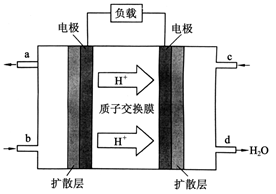

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
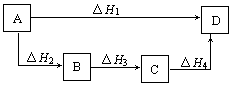
A����H1=��H2=��H3=��H 4 4 | B����H1+��H2=��H3+��H4 |
| C����H1+��H2+��H3=��H4 | D����H1=��H2+��H3+��H4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com