
A”¢B”¢C”¢D”¢E”¢F“ś±ķ6ÖÖŌŖĖŲ”£ĒėĢīæÕ£ŗ
(1)AŌŖĖŲ»łĢ¬Ō×ÓµÄ×īĶā²ćÓŠ2øöĪ“³É¶Ōµē×Ó£¬“ĪĶā²ćÓŠ2øöµē×Ó£¬ĘäŌŖĖŲ·ūŗÅĪŖ__________”£
(2)BŌŖĖŲµÄøŗŅ»¼ŪĄė×ÓŗĶCŌŖĖŲµÄÕżŅ»¼ŪĄė×ӵĵē×Ó²ć½į¹¹¶¼Óėė²ĻąĶ¬£¬BµÄŌŖĖŲ·ūŗÅĪŖ________£¬CµÄŌŖĖŲ·ūŗÅĪŖ________”£
(3)DŌŖĖŲµÄÕżČż¼ŪĄė×ÓµÄ3d¹ģµĄĪŖ°ė³äĀś£¬DµÄŌŖĖŲ·ūŗÅĪŖ________£¬Ę仳Ģ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ_________________________________________________________________”£
(4)EŌŖĖŲ»łĢ¬Ō×ÓµÄM²ćČ«³äĀś£¬N²ćƻӊ³É¶Ōµē×Ó£¬Ö»ÓŠŅ»øöĪ“³É¶Ōµē×Ó£¬EµÄŌŖĖŲ·ūŗÅĪŖ________£¬Ę仳Ģ¬Ō×ӵĵē×ÓÅŲ¼Ź½ĪŖ__________________________________”£
(5)FŌŖĖŲµÄŌ×Ó×īĶā²ćµē×ÓÅŲ¼Ź½ĪŖnsnnpn£«1£¬Ōņn£½________£»Ō×ÓÖŠÄÜĮæ×īøߵďĒ________µē×Ó”£
“š°ø””(1)C»ņO””(2)Cl””K
(3)Fe””1s22s22p63s23p63d64s2»ņ[Ar]3d64s2
(4)Cu””1s22s22p63s23p63d104s1»ņ[Ar]3d104s1
(5)2””2p
½āĪö””(1)AŌŖĖŲ»łĢ¬Ō×Ó“ĪĶā²ćÓŠ2øöµē×Ó£¬¹Ź“ĪĶā²ćĪŖK²ć£¬AŌŖĖŲÓŠ2øöµē×Ó²ć£¬ÓÉĢāŅāæÉŠ“³öĘä¹ģµĄ±ķŹ¾Ź½ĪŖ ŌņøĆŌŖĖŲŗĖĶāÓŠ6øöµē×Ó£¬ĪŖĢ¼ŌŖĖŲ£¬ĘäŌŖĖŲ·ūŗÅĪŖC£¬ĮķĶāŃõŌ×ÓĶ¬ŃłŅ²·ūŗĻŅŖĒó£¬Ęä¹ģµĄ±ķŹ¾Ź½ĪŖ
ŌņøĆŌŖĖŲŗĖĶāÓŠ6øöµē×Ó£¬ĪŖĢ¼ŌŖĖŲ£¬ĘäŌŖĖŲ·ūŗÅĪŖC£¬ĮķĶāŃõŌ×ÓĶ¬ŃłŅ²·ūŗĻŅŖĒó£¬Ęä¹ģµĄ±ķŹ¾Ź½ĪŖ
 ӣ
ӣ
(2)B£”¢C£«µÄµē×Ó²ć½į¹¹¶¼ÓėArĻąĶ¬£¬¼“ŗĖĶā¶¼ÓŠ18øöµē×Ó£¬ŌņBĪŖ17ŗÅŌŖĖŲCl£¬CĪŖ19ŗÅŌŖĖŲK”£
(3)DŌŖĖŲŌ×ÓŹ§Č„2øö4sµē×ÓŗĶ1øö3dµē×Óŗó±ä³É£«3¼ŪĄė×Ó£¬ĘäŌ×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d64s2£¬¼“ĪŖ26ŗÅŌŖĖŲĢś”£
(4)øł¾ŻĢāŅāŅŖĒó£¬Ź×ĻČŠ“³öµē×ÓÅŲ¼Ź½£ŗ
1s22s22p63s23p63d104s1£¬øĆŌŖĖŲĪŖ29ŗÅŌŖĖŲCu”£
(5)s¹ģµĄÖ»ÓŠ1øöŌ×Ó¹ģµĄ£¬¹Ź×ī¶ąÖ»ÄÜČŻÄÉ2øöµē×Ó£¬¼“n£½2£¬ĖłŅŌŌŖĖŲFµÄŌ×Ó×īĶā²ćµē×ÓÅŲ¼Ź½ĪŖ2s22p3£¬ÓÉ“ĖæÉÖŖFŹĒNŌŖĖŲ£»øł¾ŻŗĖĶāµē×ÓÅŲ¼µÄÄÜĮæ×īµĶŌĄķ£¬æÉÖŖµŖŌ×ÓµÄŗĖĶāµē×ÓÖŠµÄ2pÄܼ¶ÄÜĮæ×īøß”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠÖø¶Ø·“Ó¦µÄĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ(””””)
A£®CuČÜÓŚĻ”HNO3£ŗCu£«2H£«£«NO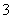 ===Cu2£«£«NO2”ü£«H2O
===Cu2£«£«NO2”ü£«H2O
B£®(NH4)2Fe(SO4)2ČÜŅŗÓė¹żĮæNaOHČÜŅŗ·“Ó¦ÖĘFe(OH)2£ŗFe2£«£«2OH£===Fe(OH)2”ż
C£®ÓĆCH3COOHČܽāCaCO3£ŗCaCO3£«2H£«===Ca2£«£«H2O£«CO2”ü
D£®ĻņNaAlO2ČÜŅŗÖŠĶØČė¹żĮæCO2ÖĘAl(OH)3£ŗCO2£«AlO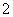 £«2H2O===Al(OH)3”ż£«HCO
£«2H2O===Al(OH)3”ż£«HCO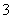
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĻÖĻóÓėĒā¼üÓŠ¹ŲµÄŹĒ(””””)
¢ŁNH3µÄČŪ”¢·Šµć±Č¢õA×åĘäĖūŌŖĖŲĒā»ÆĪļµÄøß””¢ŚŠ”·Ö×ӵē¼”¢ōČĖįæÉŅŌŗĶĖ®ŅŌČĪŅā±Č»„ČÜ””¢Ū±łµÄĆܶȱČŅŗĢ¬Ė®µÄĆܶȊ”””¢ÜÄņĖŲµÄČŪ”¢·Šµć±Č“×ĖįµÄøß””¢ŻĮŚōĒ»ł±½¼×ĖįµÄČŪ”¢·Šµć±Č¶ŌōĒ»ł±½¼×ĖįµÄµĶ””¢ŽĖ®·Ö×ÓøßĪĀĻĀŗÜĪȶØ
A£®¢Ł¢Ś¢Ū¢Ü¢Ż¢Ž B£®¢Ł¢Ś¢Ū¢Ü¢Ż
C£®¢Ł¢Ś¢Ū¢Ü D£®¢Ł¢Ś¢Ū
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ō×ÓŠņŹżĪŖ83µÄŌŖĖŲĪ»ÓŚ£ŗ¢ŁµŚ5ÖÜĘŚ£»¢ŚµŚ6ÖÜĘŚ£»¢Ū¢ōA×壻¢Ü¢õA×壻¢Ż¢ņB×壬ĘäÖŠÕżČ·µÄ×éŗĻŹĒ(””””)
A£®¢Ł¢Ü B£®¢Ś¢Ū C£®¢Ś¢Ü D£®¢Ł¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢B”¢C”¢DĪŖŌ×ÓŠņŹżŠ”ÓŚ18µÄĖÄÖÖŌŖĖŲ£ŗ
¢ŁAŌ×ӵĵē×Ó²ćŹżµČÓŚ×īĶā²ćµē×ÓŹż£»
¢ŚAÓėB“¦ÓŚĶ¬Ņ»ÖÜĘŚ£¬BÓėDæÉŠĪ³ÉĄė×Ó»ÆŗĻĪļD2B£¬øĆ»ÆŗĻĪļµÄĖ®ČÜŅŗĻŌ¼īŠŌ£»
¢ŪCµÄĄė×ÓŗĖÄŚÖŹ×ÓŹżÓėŗĖĶāµē×ÓŹżÖ®ŗĶĪŖ18£»
¢ÜA”¢C”¢DČżÖÖŌŖĖŲµÄĄė×Ó¾ßÓŠĻąĶ¬µÄµē×Ó²ćÅŲ¼”£ĶʶĻA”«Dø÷ĪŖŗĪÖÖŌŖĖŲ£¬²¢ĢīæÕ£ŗ
(1)A________£»B________£»C________£»D________”£
(2)ø÷Ō×ÓŠĪ³É¼ņµ„Ąė×ӵĵē×ÓÅŲ¼Ź½”£
(3)ĖÄÖÖŌŖĖŲĄė×Ó°ė¾¶µÄ“óŠ”Ė³ŠņĪŖ______________________________”£
(4)ÓĆĄė×Ó·½³ĢŹ½±ķŹ¾D2BĖ®ČÜŅŗ³Ź¼īŠŌµÄŌŅņ£ŗ___________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖŹ²Ć“NaČŻŅ׊Ī³É£«1¼ŪĄė×Ó£¬¶ųMg”¢AlŅ׊Ī³É£«2¼Ū”¢£«3¼ŪĄė×Ó£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾ŻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
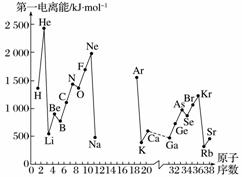
A£®µŚŅ»µēĄėÄÜI1ŹĒÖøĘųĢ¬Ō×ÓX(g)“¦ÓŚ»łĢ¬Ź±£¬Ź§Č„Ņ»øöµē×Ó³ÉĪŖĘųĢ¬ŃōĄė×ÓX£«(g)ĖłŠčµÄ×īµĶÄÜĮ攣ĻĀĶ¼ŹĒ²æ·ÖŌŖĖŲŌ×ӵĵŚŅ»µēĄėÄÜI1ĖęŌ×ÓŠņŹż±ä»ÆµÄĒśĻßĶ¼(ĘäÖŠ12ŗÅÖĮ17ŗÅŌŖĖŲµÄÓŠ¹ŲŹż¾ŻČ±Ź§)”£
B£®²»Ķ¬ŌŖĖŲµÄŌ×ÓŌŚ·Ö×ÓÄŚĪüŅżµē×ÓµÄÄÜĮ¦“óŠ”æÉÓĆŹżÖµ±ķŹ¾£¬øĆŹżÖµ³ĘĪŖµēøŗŠŌ”£Ņ»°ćČĻĪŖ£ŗČē¹ūĮ½øö³É¼üŌ×Ó¼äµÄµēøŗŠŌ²īÖµ“óÓŚ1.7£¬Ō×ÓÖ®¼äĶس£ŠĪ³ÉĄė×Ó¼ü£»Čē¹ūĮ½øö³É¼üŌ×Ó¼äµÄµēøŗŠŌ²īÖµŠ”ÓŚ1.7£¬Ķس£ŠĪ³É¹²¼Ū¼ü”£ĻĀ±ķŹĒijŠ©ŌŖĖŲµÄµēøŗŠŌÖµ£ŗ
| ŌŖĖŲ·ūŗÅ | Li | Be | B | C | O | F | Na | Al | Si | P | S | Cl |
| µēøŗŠŌÖµ | 1.0 | 1.5 | 2.0 | 2.5 | 3.5 | 4.0 | 0.9 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 |
(1)ČĻÕę·ÖĪöŠÅĻ¢AĶ¼ÖŠĶ¬ÖÜĘŚŌŖĖŲµŚŅ»µēĄėÄܵıä»Æ¹ęĀÉ£¬ĶʶĻµŚ3ÖÜĘŚNa”«ArÕā¼øÖÖŌŖĖŲÖŠ£¬AlµÄµŚŅ»µēĄėÄܵē󊔷¶Ī§ĪŖ______£¼Al£¼______(ĢīŌŖĖŲ·ūŗÅ)”£
(2)“ÓŠÅĻ¢AĶ¼ÖŠ·ÖĪöæÉÖŖ£¬Ķ¬Ņ»Ö÷×åŌŖĖŲŌ×ӵĵŚŅ»µēĄėÄÜI1µÄ±ä»Æ¹ęĀÉŹĒ________________”£
(3)ŠÅĻ¢AĶ¼ÖŠµŚŅ»µēĄėÄÜ×īŠ”µÄŌŖĖŲŌŚÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒµŚ________ÖÜĘŚµŚ________×唣
(4)øł¾Ż¶Ō½ĒĻß¹ęŌņ£¬Be”¢AlŌŖĖŲ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄŠŌÖŹĻąĖĘ£¬ĖüĆĒ¶¼¾ßÓŠ________ŠŌ£¬ĘäÖŠBe(OH)2ĻŌŹ¾ÕāÖÖŠŌÖŹµÄĄė×Ó·½³ĢŹ½ŹĒ_________________________”£
(5)Ķعż·ÖĪöµēøŗŠŌÖµµÄ±ä»Æ¹ęĀÉ£¬Č·¶ØMgŌŖĖŲµÄµēøŗŠŌÖµµÄ×īŠ”·¶Ī§________________”£
(6)Ēė¹éÄÉŌŖĖŲµÄµēøŗŠŌŗĶ½šŹōŠŌ”¢·Ē½šŹōŠŌµÄ¹ŲĻµŹĒ________________”£
(7)“ÓµēøŗŠŌ½Ē¶Č£¬ÅŠ¶ĻAlCl3ŹĒĄė×Ó»ÆŗĻĪļ»¹ŹĒ¹²¼Ū»ÆŗĻĪļ£¬Ėµ³öĄķÓɲ¢Š“³öÅŠ¶ĻµÄ·½·Ø________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖXµÄ»łĢ¬Ō×ÓL²ćµē×ÓŹżŹĒK²ćµē×ÓŹżµÄ2±¶£¬YµÄ»łĢ¬Ō×Ó×īĶā²ćµē×ÓÅŲ¼Ź½ĪŖnsnnpn£«2£¬ŌņXµÄµēøŗŠŌ±ČYµÄ________(Ģī”°“ó”±»ņ”°Š””±)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ1 200 ”ꏱ£¬ĢģČ»ĘųĶŃĮņ¹¤ŅÕÖŠ»į·¢ÉśĻĀĮŠ·“Ó¦£ŗ
H2S(g)£«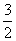 O2(g)===SO2(g)£«H2O(g)””¦¤H1
O2(g)===SO2(g)£«H2O(g)””¦¤H1
2H2S(g)£«SO2(g)===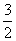 S2(g)£«2H2O(g)””¦¤H2
S2(g)£«2H2O(g)””¦¤H2
H2S(g)£«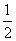 O2(g)===S(g)£«H2O(g)””¦¤H3
O2(g)===S(g)£«H2O(g)””¦¤H3
2S(g)===S2(g)””¦¤H4
Ōņ¦¤H4µÄÕżČ·±ķ“ļŹ½ĪŖ(””””)
A£®¦¤H4£½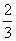 (¦¤H1£«¦¤H2£3¦¤H3)
(¦¤H1£«¦¤H2£3¦¤H3)
B£®¦¤H4£½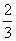 (3¦¤H3£¦¤H1£¦¤H2)
(3¦¤H3£¦¤H1£¦¤H2)
C£®¦¤H4£½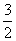 (¦¤H1£«¦¤H2£3¦¤H3)
(¦¤H1£«¦¤H2£3¦¤H3)
D£®¦¤H4£½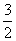 (¦¤H1£¦¤H2£3¦¤H3)
(¦¤H1£¦¤H2£3¦¤H3)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com